Genetic Testing Medical Policy – Genetics
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Factor XIII and Fibrin Clot Properties in Acute Venous Thromboembolism
International Journal of Molecular Sciences Review Factor XIII and Fibrin Clot Properties in Acute Venous Thromboembolism Michał Z ˛abczyk 1,2 , Joanna Natorska 1,2 and Anetta Undas 1,2,* 1 John Paul II Hospital, 31-202 Kraków, Poland; [email protected] (M.Z.); [email protected] (J.N.) 2 Institute of Cardiology, Jagiellonian University Medical College, 31-202 Kraków, Poland * Correspondence: [email protected]; Tel.: +48-12-614-30-04; Fax: +48-12-614-21-20 Abstract: Coagulation factor XIII (FXIII) is converted by thrombin into its active form, FXIIIa, which crosslinks fibrin fibers, rendering clots more stable and resistant to degradation. FXIII affects fibrin clot structure and function leading to a more prothrombotic phenotype with denser networks, characterizing patients at risk of venous thromboembolism (VTE). Mechanisms regulating FXIII activation and its impact on fibrin structure in patients with acute VTE encompassing pulmonary embolism (PE) or deep vein thrombosis (DVT) are poorly elucidated. Reduced circulating FXIII levels in acute PE were reported over 20 years ago. Similar observations indicating decreased FXIII plasma activity and antigen levels have been made in acute PE and DVT with their subsequent increase after several weeks since the index event. Plasma fibrin clot proteome analysis confirms that clot-bound FXIII amounts associated with plasma FXIII activity are decreased in acute VTE. Reduced FXIII activity has been associated with impaired clot permeability and hypofibrinolysis in acute PE. The current review presents available studies on the role of FXIII in the modulation of fibrin clot properties during acute PE or DVT and following these events. -
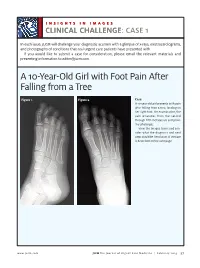
A 10-Year-Old Girl with Foot Pain After Falling from a Tree
INSIGHTS IN IMAGES CLINICAL CHALLENGECHALLENGE: CASE 1 In each issue, JUCM will challenge your diagnostic acumen with a glimpse of x-rays, electrocardiograms, and photographs of conditions that real urgent care patients have presented with. If you would like to submit a case for consideration, please email the relevant materials and presenting information to [email protected]. A 10-Year-Old Girl with Foot Pain After Falling from a Tree Figure 1. Figure 2. Case A 10-year-old girl presents with pain after falling from a tree, landing on her right foot. On examination, the pain emanates from the second through fifth metatarsals and proxi- mal phalanges. View the images taken and con- sider what the diagnosis and next steps would be. Resolution of the case is described on the next page. www.jucm.com JUCM The Journal of Urgent Care Medicine | February 2019 37 INSIGHTS IN IMAGES: CLINICAL CHALLENGE THE RESOLUTION Figure 1. Mediastinal air Figure 1. Differential Diagnosis Pearls for Urgent Care Management and Ⅲ Fracture of the distal fourth metatarsal Considerations for Transfer Ⅲ Plantar plate disruption Ⅲ Emergent transfer should be considered with associated neu- Ⅲ Sesamoiditis rologic deficit, compartment syndrome, open fracture, or vas- Ⅲ Turf toe cular compromise Ⅲ Referral to an orthopedist is warranted in the case of an in- Diagnosis tra-articular fracture, or with Lisfranc ligament injury or ten- Angulation of the distal fourth metatarsal metaphyseal cortex derness over the Lisfranc ligament and hairline lucency consistent with fracture. Acknowledgment: Images courtesy of Teleradiology Associates. Learnings/What to Look for Ⅲ Proximal metatarsal fractures are most often caused by crush- ing or direct blows Ⅲ In athletes, an axial load placed on a plantar-flexed foot should raise suspicion of a Lisfranc injury 38 JUCM The Journal of Urgent Care Medicine | February 2019 www.jucm.com INSIGHTS IN IMAGES CLINICAL CHALLENGE: CASE 2 A 55-Year-Old Man with 3 Hours of Epigastric Pain 55 years PR 249 QRSD 90 QT 471 QTc 425 AXES P 64 QRS -35 T 30 Figure 1. -

Reframing Psychiatry for Precision Medicine
Reframing Psychiatry for Precision Medicine Elizabeth B Torres 1,2,3* 1 Rutgers University Department of Psychology; [email protected] 2 Rutgers University Center for Cognitive Science (RUCCS) 3 Rutgers University Computer Science, Center for Biomedicine Imaging and Modelling (CBIM) * Correspondence: [email protected]; Tel.: (011) +858-445-8909 (E.B.T) Supplementary Material Sample Psychological criteria that sidelines sensory motor issues in autism: The ADOS-2 manual [1, 2], under the “Guidelines for Selecting a Module” section states (emphasis added): “Note that the ADOS-2 was developed for and standardized using populations of children and adults without significant sensory and motor impairments. Standardized use of any ADOS-2 module presumes that the individual can walk independently and is free of visual or hearing impairments that could potentially interfere with use of the materials or participation in specific tasks.” Sample Psychiatric criteria from the DSM-5 [3] that does not include sensory-motor issues: A. Persistent deficits in social communication and social interaction across multiple contexts, as manifested by the following, currently or by history (examples are illustrative, not exhaustive, see text): 1. Deficits in social-emotional reciprocity, ranging, for example, from abnormal social approach and failure of normal back-and-forth conversation; to reduced sharing of interests, emotions, or affect; to failure to initiate or respond to social interactions. 2. Deficits in nonverbal communicative behaviors used for social interaction, ranging, for example, from poorly integrated verbal and nonverbal communication; to abnormalities in eye contact and body language or deficits in understanding and use of gestures; to a total lack of facial expressions and nonverbal communication. -

CADASIL Testing
Lab Management Guidelines V1.0.2020 CADASIL Testing MOL.TS.144.A v1.0.2020 Introduction CADASIL testing is addressed by this guideline. Procedures addressed The inclusion of any procedure code in this table does not imply that the code is under management or requires prior authorization. Refer to the specific Health Plan's procedure code list for management requirements. Procedures addressed by this Procedure codes guideline NOTCH3 Known Familial Mutation 81403 Analysis NOTCH3 Targeted Sequencing 81406 NOTCH3 Deletion/Duplication Analysis 81479 What is CADASIL Definition CADASIL (Cerebral Autosomal Dominant Arteriopathy with Subcortical Infarcts and Leukoencephalopathy) is an adult-onset form of cerebrovascular disease. There are no generally accepted clinical diagnostic criteria for CADASIL and symptoms vary among affected individuals. Signs and symptoms Typical signs and symptoms include1,2,3 Transient ischemic attacks and ischemic stroke, occurs at a mean age of 47 years (age range 20-70 years), in most cases without conventional vascular risk factors cognitive disturbance, primarily affecting executive function, may start as early as age 35 years psychiatric or behavioral abnormalities migraine with aura, occurs with a mean age of onset of 30 years (age range 6-48 years), and Less common symptoms include: © 2020 eviCore healthcare. All Rights Reserved. 1 of 7 400 Buckwalter Place Boulevard, Bluffton, SC 29910 (800) 918-8924 www.eviCore.com Lab Management Guidelines V1.0.2020 recurrent seizures with onset in middle age, usually secondary to stroke acute encephalopathy, with a mean age of onset of 42 years Life expectancy for men with CADASIL is reduced by approximately five years and for women by 1 to 2 years.4 Diagnosis Brain Magnetic Resonance Imaging (MRI) findings include T2-signal-abnormalities in the white matter of the temporal pole and T2-signal-abnormalities in the external capsule and corpus callosum.1,2 CADASIL is suspected in an individual with the clinical signs and MRI findings. -

Comparative Proteomic Analysis Revealed Complex Responses To
www.nature.com/scientificreports OPEN Comparative proteomic analysis revealed complex responses to classical/novel duck reovirus Received: 12 January 2018 Accepted: 20 June 2018 infections in Cairna moschata Published: xx xx xxxx Tao Yun, Jionggang Hua, Weicheng Ye, Bin Yu, Liu Chen, Zheng Ni & Cun Zhang Duck reovirus (DRV) is an typical aquatic bird pathogen belonging to the Orthoreovirus genus of the Reoviridae family. Reovirus causes huge economic losses to the duck industry. Although DRV has been identifed and isolated long ago, the responses of Cairna moschata to classical/novel duck reovirus (CDRV/NDRV) infections are largely unknown. To investigate the relationship of pathogenesis and immune response, proteomes of C. moschata liver cells under the C/NDRV infections were analyzed, respectively. In total, 5571 proteins were identifed, among which 5015 proteins were quantifed. The diferential expressed proteins (DEPs) between the control and infected liver cells displayed diverse biological functions and subcellular localizations. Among the DEPs, most of the metabolism-related proteins were down-regulated, suggesting a decrease in the basal metabolisms under C/NDRV infections. Several important factors in the complement, coagulation and fbrinolytic systems were signifcantly up-regulated by the C/NDRV infections, indicating that the serine protease-mediated innate immune system might play roles in the responses to the C/NDRV infections. Moreover, a number of molecular chaperones were identifed, and no signifcantly changes in their abundances were observed in the liver cells. Our data may give a comprehensive resource for investigating the regulation mechanism involved in the responses of C. moschata to the C/NDRV infections. Duck reovirus (DRV), a member of the genus Orthoreovirus in the family Reoviridae, is a fatal aquatic bird patho- gen1. -

The National Economic Burden of Rare Disease Study February 2021
Acknowledgements This study was sponsored by the EveryLife Foundation for Rare Diseases and made possible through the collaborative efforts of the national rare disease community and key stakeholders. The EveryLife Foundation thanks all those who shared their expertise and insights to provide invaluable input to the study including: the Lewin Group, the EveryLife Community Congress membership, the Technical Advisory Group for this study, leadership from the National Center for Advancing Translational Sciences (NCATS) at the National Institutes of Health (NIH), the Undiagnosed Diseases Network (UDN), the Little Hercules Foundation, the Rare Disease Legislative Advocates (RDLA) Advisory Committee, SmithSolve, and our study funders. Most especially, we thank the members of our rare disease patient and caregiver community who participated in this effort and have helped to transform their lived experience into quantifiable data. LEWIN GROUP PROJECT STAFF Grace Yang, MPA, MA, Vice President Inna Cintina, PhD, Senior Consultant Matt Zhou, BS, Research Consultant Daniel Emont, MPH, Research Consultant Janice Lin, BS, Consultant Samuel Kallman, BA, BS, Research Consultant EVERYLIFE FOUNDATION PROJECT STAFF Annie Kennedy, BS, Chief of Policy and Advocacy Julia Jenkins, BA, Executive Director Jamie Sullivan, MPH, Director of Policy TECHNICAL ADVISORY GROUP Annie Kennedy, BS, Chief of Policy & Advocacy, EveryLife Foundation for Rare Diseases Anne Pariser, MD, Director, Office of Rare Diseases Research, National Center for Advancing Translational Sciences (NCATS), National Institutes of Health Elisabeth M. Oehrlein, PhD, MS, Senior Director, Research and Programs, National Health Council Christina Hartman, Senior Director of Advocacy, The Assistance Fund Kathleen Stratton, National Academies of Science, Engineering and Medicine (NASEM) Steve Silvestri, Director, Government Affairs, Neurocrine Biosciences Inc. -

Genetic Mechanisms of Disease in Children: a New Look
Genetic Mechanisms of Disease in Children: A New Look Laurie Demmer, MD Tufts Medical Center and the Floating Hospital for Children Boston, MA Traditionally genetic disorders have been linked to the ‘one gene-one protein-one disease’ hypothesis. However recent advances in the field of molecular biology and biotechnology have afforded us the opportunity to greatly expand our knowledge of genetics, and we now know that the mechanisms of inherited disorders are often significantly more complex, and consequently, much more intriguing, than originally thought. Classical mendelian disorders with relatively simple genetic mechanisms do exist, but turn out to be far more rare than originally thought. All patients with sickle cell disease for example, carry the same A-to-T point mutation in the sixth codon of the beta globin gene. This results in a glutamate to valine substitution which changes the shape and the function of the globin molecule in a predictable way. Similarly, all patients with achondroplasia have a single base pair substitution at nucleotide #1138 of the FGFR3 gene. On the other hand, another common inherited disorder, cystic fibrosis, is known to result from changes in a specific transmembrane receptor (CFTR), but over 1000 different disease-causing mutations have been reported in this single gene. Since most commercial labs only test for between 23-100 different mutations, interpreting CFTR mutation testing is significantly complicated by the known risk of false negative results. Many examples of complex, or non-Mendelian, inheritance are now known to exist and include disorders of trinucleotide repeats, errors in imprinting, and gene dosage effects. -

Sensory Deficits in a Mouse Model of Christianson Syndrome Tarheen
Sensory Deficits in a Mouse Model of Christianson Syndrome Tarheen Fatima Department of Physiology McGill University, Montreal April 2019 A thesis submitted to McGill University in partial fulfillment of the requirements of the degree of Master of Science. © Tarheen Fatima 2019 Abstract Christianson Syndrome (CS) is a recently characterized X-linked neurodevelopmental disorder caused by loss-of-function mutations in the gene slc9a6, encoding the endosomal Na+/H+ Exchanger 6 (NHE6). The disorder is associated with developmental delay, intellectual disability, loss of motor coordination, mutism, ataxia, epilepsy, as well as autistic features. In addition to these symptoms, CS patients exhibit elevated pain thresholds to noxious stimuli as well as discomfort at normally innocuous stimuli. The underlying causes of these sensory deficits are yet to be determined. Hence, this study aims at understanding how loss-of-function of NHE6 affects transmission and processing of pain. To this end, we examined the expression of NHE6 in peripheral and central neurons implicated in sensation and interpretation of pain. Additionally, we characterized the nociceptive behaviour of NHE6 knockout (KO) mice using a battery of behaviour tests. Our immunohistochemical experiments demonstrate that NHE6 is highly expressed in nociceptive, small-diameter dorsal root ganglia (DRG) neurons. Moreover, mice lacking NHE6 display decreased responses to noxious mechanical, thermal and chemical stimuli but are more responsive to noxious cold than wild-type littermates. Interestingly, immunohistochemical characterization of DRG tissue from aged NHE6 null mice indicates a decrease in some neuronal subsets suggesting cell death. Finally, using light brush-induced Fos activation in the dorsal horn, we found that the spinal processing of innocuous stimuli is not different between wildtype and NHE6 knockout mice. -

Crouzon Syndrome Genetic and Intervention Review
Journal of Oral Biology and Craniofacial Research 9 (2019) 37–39 Contents lists available at ScienceDirect Journal of Oral Biology and Craniofacial Research journal homepage: www.elsevier.com/locate/jobcr Crouzon syndrome: Genetic and intervention review ∗ T N.M. Al-Namnama, , F. Haririb, M.K. Thongc, Z.A. Rahmanb a Department of Oral Biology, Faculty of Dentistry, University of MAHSA, 42610, Jenjarum, Selangor, Malaysia b Department of Oro-Maxillofacial Clinical Science, Faculty of Dentistry, University of Malaya, 50603, Kuala Lumpur, Malaysia c Department of Paediatrics, Faculty of Medicine, University of Malaya, 50603, Kuala Lumpur, Malaysia ARTICLE INFO ABSTRACT Keywords: Crouzon syndrome exhibits considerable phenotypic heterogeneity, in the aetiology of which genetics play an Crouzon syndrome important role. FGFR2 mediates extracellular signals into cells and the mutations in the FGFR2 gene cause this Molecular pathology syndrome occurrence. Activated FGFs/FGFR2 signaling disrupts the balance of differentiation, cell proliferation, Genetic phenotype and apoptosis via its downstream signal pathways. However, very little is known about the cellular and mole- cular factors leading to severity of this phenotype. Revealing the molecular pathology of craniosynostosis will be a great value for genetic counselling, diagnosis, prognosis and early intervention programs. This mini-review summarizes the fundamental and recent scientific literature on genetic disorder of Crouzon syndrome and presents a graduated strategy for the genetic approach, diagnosis and the management of this complex cra- niofacial defect. 1. Introduction known. CS commonly starts at the first three years of life.4 Craniosy- nostosis can be suspected during antenatal stage via ultrasound scan Craniosynostosis is a birth defect characterized by premature fusion otherwise is often detected at birth from its classic crouzonoid features of one or more of the calvarial sutures before the completion of brain of the newborn. -

Advances in Understanding the Genetics of Syndromes Involving Congenital Upper Limb Anomalies
Review Article Page 1 of 10 Advances in understanding the genetics of syndromes involving congenital upper limb anomalies Liying Sun1#, Yingzhao Huang2,3,4#, Sen Zhao2,3,4, Wenyao Zhong1, Mao Lin2,3,4, Yang Guo1, Yuehan Yin1, Nan Wu2,3,4, Zhihong Wu2,3,5, Wen Tian1 1Hand Surgery Department, Beijing Jishuitan Hospital, Beijing 100035, China; 2Beijing Key Laboratory for Genetic Research of Skeletal Deformity, Beijing 100730, China; 3Medical Research Center of Orthopedics, Chinese Academy of Medical Sciences, Beijing 100730, China; 4Department of Orthopedic Surgery, 5Department of Central Laboratory, Peking Union Medical College Hospital, Peking Union Medical College and Chinese Academy of Medical Sciences, Beijing 100730, China Contributions: (I) Conception and design: W Tian, N Wu, Z Wu, S Zhong; (II) Administrative support: All authors; (III) Provision of study materials or patients: All authors; (IV) Collection and assembly of data: Y Huang; (V) Data analysis and interpretation: L Sun; (VI) Manuscript writing: All authors; (VII) Final approval of manuscript: All authors. Correspondence to: Wen Tian. Hand Surgery Department, Beijing Jishuitan Hospital, Beijing 100035, China. Email: [email protected]. Abstract: Congenital upper limb anomalies (CULA) are a common birth defect and a significant portion of complicated syndromic anomalies have upper limb involvement. Mostly the mortality of babies with CULA can be attributed to associated anomalies. The cause of the majority of syndromic CULA was unknown until recently. Advances in genetic and genomic technologies have unraveled the genetic basis of many syndromes- associated CULA, while at the same time highlighting the extreme heterogeneity in CULA genetics. Discoveries regarding biological pathways and syndromic CULA provide insights into the limb development and bring a better understanding of the pathogenesis of CULA. -

2018 Etiologies by Frequencies
2018 Etiologies in Order of Frequency by Category Hereditary Syndromes and Disorders Count CHARGE Syndrome 958 Down syndrome (Trisomy 21 syndrome) 308 Usher I syndrome 252 Stickler syndrome 130 Dandy Walker syndrome 119 Cornelia de Lange 102 Goldenhar syndrome 98 Usher II syndrome 83 Wolf-Hirschhorn syndrome (Trisomy 4p) 68 Trisomy 13 (Trisomy 13-15, Patau syndrome) 60 Pierre-Robin syndrome 57 Moebius syndrome 55 Trisomy 18 (Edwards syndrome) 52 Norrie disease 38 Leber congenital amaurosis 35 Chromosome 18, Ring 18 31 Aicardi syndrome 29 Alstrom syndrome 27 Pfieffer syndrome 27 Treacher Collins syndrome 27 Waardenburg syndrome 27 Marshall syndrome 25 Refsum syndrome 21 Cri du chat syndrome (Chromosome 5p- synd) 16 Bardet-Biedl syndrome (Laurence Moon-Biedl) 15 Hurler syndrome (MPS I-H) 15 Crouzon syndrome (Craniofacial Dysotosis) 13 NF1 - Neurofibromatosis (von Recklinghausen dis) 13 Kniest Dysplasia 12 Turner syndrome 11 Usher III syndrome 10 Cockayne syndrome 9 Apert syndrome/Acrocephalosyndactyly, Type 1 8 Leigh Disease 8 Alport syndrome 6 Monosomy 10p 6 NF2 - Bilateral Acoustic Neurofibromatosis 6 Batten disease 5 Kearns-Sayre syndrome 5 Klippel-Feil sequence 5 Hereditary Syndromes and Disorders Count Prader-Willi 5 Sturge-Weber syndrome 5 Marfan syndrome 3 Hand-Schuller-Christian (Histiocytosis X) 2 Hunter Syndrome (MPS II) 2 Maroteaux-Lamy syndrome (MPS VI) 2 Morquio syndrome (MPS IV-B) 2 Optico-Cochleo-Dentate Degeneration 2 Smith-Lemli-Opitz (SLO) syndrome 2 Wildervanck syndrome 2 Herpes-Zoster (or Hunt) 1 Vogt-Koyanagi-Harada -

My Beloved Neutrophil Dr Boxer 2014 Neutropenia Family Conference
The Beloved Neutrophil: Its Function in Health and Disease Stem Cell Multipotent Progenitor Myeloid Lymphoid CMP IL-3, SCF, GM-CSF CLP Committed Progenitor MEP GMP GM-CSF, IL-3, SCF EPO TPO G-CSF M-CSF IL-5 IL-3 SCF RBC Platelet Neutrophil Monocyte/ Basophil B-cells Macrophage Eosinophil T-Cells Mast cell NK cells Mature Cell Dendritic cells PRODUCTION AND KINETICS OF NEUTROPHILS CELLS % CELLS TIME Bone Marrow: Myeloblast 1 7 - 9 Mitotic Promyelocyte 4 Days Myelocyte 16 Maturation/ Metamyelocyte 22 3 – 7 Storage Band 30 Days Seg 21 Vascular: Peripheral Blood Seg 2 6 – 12 hours 3 Marginating Pool Apoptosis and ? Tissue clearance by 0 – 3 macrophages days PHAGOCYTOSIS 1. Mobilization 2. Chemotaxis 3. Recognition (Opsonization) 4. Ingestion 5. Degranulation 6. Peroxidation 7. Killing and Digestion 8. Net formation Adhesion: β 2 Integrins ▪ Heterodimer of a and b chain ▪ Tight adhesion, migration, ingestion, co- stimulation of other PMN responses LFA-1 Mac-1 (CR3) p150,95 a2b2 a CD11a CD11b CD11c CD11d b CD18 CD18 CD18 CD18 Cells All PMN, Dendritic Mac, mono, leukocytes mono/mac, PMN, T cell LGL Ligands ICAMs ICAM-1 C3bi, ICAM-3, C3bi other other Fibrinogen other GRANULOCYTE CHEMOATTRACTANTS Chemoattractants Source Activators Lipids PAF Neutrophils C5a, LPS, FMLP Endothelium LTB4 Neutrophils FMLP, C5a, LPS Chemokines (a) IL-8 Monocytes, endothelium LPS, IL-1, TNF, IL-3 other cells Gro a, b, g Monocytes, endothelium IL-1, TNF other cells NAP-2 Activated platelets Platelet activation Others FMLP Bacteria C5a Activation of complement Other Important Receptors on PMNs ñ Pattern recognition receptors – Detect microbes - Toll receptor family - Mannose receptor - bGlucan receptor – fungal cell walls ñ Cytokine receptors – enhance PMN function - G-CSF, GM-CSF - TNF Receptor ñ Opsonin receptors – trigger phagocytosis - FcgRI, II, III - Complement receptors – ñ Mac1/CR3 (CD11b/CD18) – C3bi ñ CR-1 – C3b, C4b, C3bi, C1q, Mannose binding protein From JG Hirsch, J Exp Med 116:827, 1962, with permission.