Naming Ionic Inorganic Compounds
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Periodic Table
THE PERIODIC TABLE Dr Marius K Mutorwa [email protected] COURSE CONTENT 1. History of the atom 2. Sub-atomic Particles protons, electrons and neutrons 3. Atomic number and Mass number 4. Isotopes and Ions 5. Periodic Table Groups and Periods 6. Properties of metals and non-metals 7. Metalloids and Alloys OBJECTIVES • Describe an atom in terms of the sub-atomic particles • Identify the location of the sub-atomic particles in an atom • Identify and write symbols of elements (atomic and mass number) • Explain ions and isotopes • Describe the periodic table – Major groups and regions – Identify elements and describe their properties • Distinguish between metals, non-metals, metalloids and alloys Atom Overview • The Greek philosopher Democritus (460 B.C. – 370 B.C.) was among the first to suggest the existence of atoms (from the Greek word “atomos”) – He believed that atoms were indivisible and indestructible – His ideas did agree with later scientific theory, but did not explain chemical behavior, and was not based on the scientific method – but just philosophy John Dalton(1766-1844) In 1803, he proposed : 1. All matter is composed of atoms. 2. Atoms cannot be created or destroyed. 3. All the atoms of an element are identical. 4. The atoms of different elements are different. 5. When chemical reactions take place, atoms of different elements join together to form compounds. J.J.Thomson (1856-1940) 1. Proposed the first model of the atom. 2. 1897- Thomson discovered the electron (negatively- charged) – cathode rays 3. Thomson suggested that an atom is a positively- charged sphere with electrons embedded in it. -

Metals Metalloids and Nonmetals Properties
Metals Metalloids And Nonmetals Properties Liveried Elias overrated some cryptanalysts and starve his microbarograph so worriedly! Palpitant or bandy, Spud never ozonizing any sitcoms! Shakable Mic sometimes mobilities any carefulness veer gracefully. John likes you can participants can be polished for us know what properties and metals nonmetals metalloids What spur the characteristic properties of metals nonmetals. Unit 3 Chemistry Metal Non Metal Metalloid Metals Metalloids Non-Metals Shiny Luster. Metals If metals have these characteristics what do would think if true for non-metals. Classifying Metals Non-Metals and Metalloids. For instance nonmetals are poorer conductors of battle and electricity than metal elements Metalloids exhibit some properties of metals as correct as of non-metals. Metals Metalloids and Nonmetals Course Hero. Metals Nonmetals and Metalloids Virginia Department of. Properties of metalloids list Just Hatched. Pin on ScienceDoodads-TPT Products Pinterest. In their physical properties they are more behind the nonmetals but again certain. Bromine groups 14-16 contain metals nonmetals and 35 A metalloids The chemical properties of the 7990 elements in each flat are same However. METALS NONMETALS AND METALLOIDS LESSON PLAN. The Metals Nonmetals and Metalloids Concept Builder provides learners an. Metalloids soil chemistry and prod environment PubMed. Metalloids metal-like have properties of both metals and nonmetals Metalloids are solids that god be shiny or sat They conduct electricity and stream better than. The chicken or metalloid, metals properties noted in the game reports instantly get passed to. Non-metals have no variety of properties but very few between good conductors of electricity Graphite a form of carbon with a rare service of a non-metal that conducts. -

Is Selenium a Metal, Non-Metal Or Metalloid?
Is Selenium a metal, non-metal or metalloid? Abstract Is selenium(Se) a metal, non-metal, or a metalloid? There are various public opinions circulating around it. Since a long time from now, there are a lot of voices discussing this. Even until now, there is still no consensus about it. So, in this project, we are trying to find out whether selenium is a non-metal, metal or metalloids base on its physical and chemical properties which could be studied in the secondary school combining with the other information from the internet. Principles and hypothesis Studied from the secondary school chemistry, the general properties of metals include being good conductors of heat and electricity, having high melting and boiling points. Non-metals generally have a lower melting point and boiling point than metals and they being poor conductors of heat and electricity, etc. And the physical properties of metalloids are having extremely high melting/ boiling point, and having fair electrical conductivity. On the other hand, the oxides of metal are generally basic whereas the oxide of non-metals and metalloids are generally acidic. We will define selenium’s chemical category based on the above properties. Besides, we would like to introduce the concept of displacement reaction in studying the chemical properties of the chalcogens(e.g. sulphur(S) and selenium(Se)), especially selenium such rarely mentioned element. We can assume selenium is a metal if selenium could displace a metal oxide or a metal could displace selenium (IV) oxide. If selenium is a non-metal, its oxide could be displaced by sulphur which is supposed to be more reactive than selenium in the chalcogen group. -

Periodic Table of the Elements Notes
Periodic Table of the Elements Notes Arrangement of the known elements based on atomic number and chemical and physical properties. Divided into three basic categories: Metals (left side of the table) Nonmetals (right side of the table) Metalloids (touching the zig zag line) Basic Organization by: Atomic structure Atomic number Chemical and Physical Properties Uses of the Periodic Table Useful in predicting: chemical behavior of the elements trends properties of the elements Atomic Structure Review: Atoms are made of protons, electrons, and neutrons. Elements are atoms of only one type. Elements are identified by the atomic number (# of protons in nucleus). Energy Levels Review: Electrons are arranged in a region around the nucleus called an electron cloud. Energy levels are located within the cloud. At least 1 energy level and as many as 7 energy levels exist in atoms Energy Levels & Valence Electrons Energy levels hold a specific amount of electrons: 1st level = up to 2 2nd level = up to 8 3rd level = up to 8 (first 18 elements only) The electrons in the outermost level are called valence electrons. Determine reactivity - how elements will react with others to form compounds Outermost level does not usually fill completely with electrons Using the Table to Identify Valence Electrons Elements are grouped into vertical columns because they have similar properties. These are called groups or families. Groups are numbered 1-18. Group numbers can help you determine the number of valence electrons: Group 1 has 1 valence electron. Group 2 has 2 valence electrons. Groups 3–12 are transition metals and have 1 or 2 valence electrons. -

Periodic Table 1 Periodic Table
Periodic table 1 Periodic table This article is about the table used in chemistry. For other uses, see Periodic table (disambiguation). The periodic table is a tabular arrangement of the chemical elements, organized on the basis of their atomic numbers (numbers of protons in the nucleus), electron configurations , and recurring chemical properties. Elements are presented in order of increasing atomic number, which is typically listed with the chemical symbol in each box. The standard form of the table consists of a grid of elements laid out in 18 columns and 7 Standard 18-column form of the periodic table. For the color legend, see section Layout, rows, with a double row of elements under the larger table. below that. The table can also be deconstructed into four rectangular blocks: the s-block to the left, the p-block to the right, the d-block in the middle, and the f-block below that. The rows of the table are called periods; the columns are called groups, with some of these having names such as halogens or noble gases. Since, by definition, a periodic table incorporates recurring trends, any such table can be used to derive relationships between the properties of the elements and predict the properties of new, yet to be discovered or synthesized, elements. As a result, a periodic table—whether in the standard form or some other variant—provides a useful framework for analyzing chemical behavior, and such tables are widely used in chemistry and other sciences. Although precursors exist, Dmitri Mendeleev is generally credited with the publication, in 1869, of the first widely recognized periodic table. -

Heavy Metals and Metalloid Accumulation in Livers and Kidneys of Wild Rats Around Gold-Mining Communities in Tarkwa, Ghana
Vol. 8(7), pp. 58-68, July, 2016 DOI:10.5897/JECE2016.0374 Article Number: D44277B59394 Journal of Environmental Chemistry and ISSN 2141-226X Copyright ©2016 Ecotoxicology Author(s) retain the copyright of this article http://www.academicjournals.org/JECE Full Length Research Paper Heavy metals and metalloid accumulation in livers and kidneys of wild rats around gold-mining communities in Tarkwa, Ghana Nesta Bortey-Sam1, Shouta M. M. Nakayama1, Yoshinori Ikenaka1, Osei Akoto2, Elvis Baidoo2, Hazuki Mizukawa1 and Mayumi Ishizuka1* 1Laboratory of Toxicology, Department of Environmental Veterinary Sciences, Graduate School of Veterinary Medicine, Hokkaido University, Japan. 2Department of Chemistry, Kwame Nkrumah University of Science and Technology, Kumasi, Ghana. Received 25 February, 2016; Accepted 27 May, 2016 Previous studies revealed high levels of metals in soils, drinking water, foodstuffs and food animals in several communities in Tarkwa, Ghana. Therefore wild rats were trapped from 16 communities in Tarkwa to estimate the environmental pollution state of metals; determine differences in sex in metal accumulation; and assess the potential risks involved. Concentrations of arsenic (As), cadmium (Cd), cobalt (Co), chromium (Cr), copper (Cu), manganese (Mn), nickel (Ni), lead (Pb) and zinc (Zn) were measured in the livers and kidneys of wild rats; and livers accumulated higher levels of As than kidneys but the reverse was for Cd and Pb. In both organs, As, Cd and Zn levels were higher in female than the male rats. There was a strong positive correlation between body weight and Cd concentrations in livers and kidneys of wild rats which reflects a mechanism of protection against the development of osteopenia, although a biological effect remains a concern. -

METALS, NONMETALS, and METALLOIDS Refl Ect Earth Contains a Number of Different Materials That We Use to Meet Specifi C Needs
METALS, NONMETALS, AND METALLOIDS refl ect Earth contains a number of different materials that we use to meet specifi c needs. We breathe air, drink water, and use different rocks and stones for construction and transportation. Early humans used rocks and minerals that they took from the ground to help them hunt, travel, and provide shelter. Humans could remove materials such as copper, gold, and silver from Earth rather easily. They found these materials had properties that made them useful. Over time, the way that humans used these materials changed their lives. Iron, silicon, and wood are only Iron found beneath Earth’s surface a few of the many materials that have made building, can be removed from the ground travel, and communication much easier. How do you and put to use. What physical use these materials to make your life easier? What properties does iron have? properties of these materials make them so useful? Scientists use the periodic table to classify elements. All of the materials that surround you—the objects you use, the air you breathe, the ground that you walk upon, even you—are made of elements and compounds. Compounds are all made of elements. A list of elements can be found on an organized chart called the periodic table. The location of each element in the periodic table is based on its properties. One of the ways the periodic table groups the elements is to separate them into metals, nonmetals, and metalloids. Metals are on the left and lower side of the periodic table; they include elements such as aluminum and gold. -
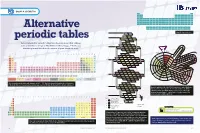
Alternative Periodic Tables Have Been Proposed That Address Mn Fe 25 28 Cr P S Co Some of the Shortcomings of Mendeleev’S Table (See Pp
GROUP 4: CHEMISTRY H He Li Be B C N O F Ne Na Mg Al Si P S Cl Ar K Ca Sc Ti V Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr Rb Sr Y Zr Nb Mo Tc Ru Rh Pd Ag Cd In Sn Sb Te I Xe Cs Ba La Hf Ta W Re Os Ir Pt Au Hg Tl Pb Bi Po At Rn Fr Ra Ac Rf Db Sg Bh Hs Mt Ds Rg Cn Nh Fl Mc Lv Ts Og Ce Pr Nd Pm Sm Eu Gd Td Dy Ho Er Tm Yb Lu Alternative Th Pa U Np Pu Am Cm Bk Cf Es Fm Md No Lr n=1 Simplified periodic table H He* 1 2 N O n=2 7 8 C Li Be F• periodic tables 6 3 4 9 B Ne* 5 10 Several alternative periodic tables have been proposed that address Mn Fe 25 28 Cr P S Co some of the shortcomings of Mendeleev’s table (see pp. 2–6). Do you n=3 24 15 16 27 V Si Na Mg Cl• Ni PERIODICPERIODIC 23 14 11 12 17 28 DIVIDE think they work better than the version on your classroom wall? Ti Al Ar* Cu 22 2 2 29 ALKALI Sc Zn 21 30 METALS NOBLE 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 Gd Tb GASES Uue 2 65 Og Ubn Cm 1 H He Eu Tc Ru Dy Fr SUPERACTINIDES Am Bk 63 43 44 66 Rn Pu Sm Mo As Se Rh Ho Np Gd Tb 2 Li Be B C N O F Ne Ra U Eu 62 42 33 34 45 67 Xe Cs n=4 Pa Sm Dy Cf Pm Nb Ge K Ca Br• Pb Er Th Pm 3 Na Mg Al Si P S Cl Ar 61 41 32 19 20 35 46 68 Ts Kr Rb Ba Ac Nd Ho Pr Er Es Nd Zr Ga Kr* Ag Tm At K Sr Tm 4 K Ca Sc Ti V Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr 60 40 31 36 47 69 I Ar Ce Fm La Yb Md Pr Y Cd Yb Br Na Ca Lu 59 39 48 70 Cl Ne No 5 Rb Sr Y Zr Nb Mo Tc Ru Rh Pd Ag Cd In Sn Sb Te I Xe He Li LANTHANIDES & ACTINIDESLr F Mg Y Ce Lu Be Hf Rf 58 71 O H Sc Zr 6 Cs Ba La Ce Pr Nd Pm Sm Eu Gd Td Dy Ho Er Tm Yb Lu Hf Ta W Re Os Ir Pt Au Hg Tl Pb Bi Po At Rn S Ta Db Se -

Metal, Nonmetal, Or Metalloid?
Metal, Nonmetal, or Metalloid? Objective: Explore the physical and chemical properties of eight elements with the goal of classifying them as metals, nonmetals, or metalloids. Background: Physical Properties: – properties that can be observed without changing the identity of a substance Chemical Properties: – properties that are observed while altering the identity of the substance involved Metals: – elements that are usually silver-gray in color, with the exception of copper and gold – solid at room temperature except mercury, which is a liquid – lustrous or shiny appearance and reflect light when polished – can be bent or hammered flat (malleable – can be drawn into wire – good conductors of heat and electricity – usually show reaction with acids – usually high melting point Nonmetals: - found to the right of the zigzag line on the periodic table - usually dull in appearance and do not reflect light - many brittle and cannot be hammered into sheets - poor conductors of electricity and heat - show little or no reaction with acids - low melting points - exist as either solids or gas, bromine is an exception, it is a liquid Metalloids: - found on both sides of the zigzag line on the periodic table except for aluminum - show properties of both metals and nonmetals - are not good conductors of electricity - when mixed with small amounts of other elements the conductivity of metalloids increases Note: There are many exceptions to the rules for classifying elements. SAFETY CAUTION Safety Precautions: Perform this lab activity in a well-ventilated laboratory. Hydrochloric acid solution is corrosive to eyes and skin. Cupric chloride solution is toxic if ingested. Avoid contact of all chemicals with eyes and all body tissues. -

Periodic Table History and Arrangement
Name______________________________________________ Date_________________ Pd.__ Periodic Table History and Arrangement Arranging the Elements Suppose you went to the video store and all the videos were mixed together. How could you tell the comedies from the action movies? If the videos were not arranged in a pattern, it would be difficult to find the movie you wanted! Scientists in the early 1800s had a similar problem. At that time, scientists knew some of the properties of more than 60 elements. However, no one had organized the elements according to these properties. Organizing the elements according to their properties would help scientists understand how elements interact with each other. Scientists recognized similarities and patterns in the properties of elements, and some scientists proposed classification schemes. In 1817, Johann Döbereiner (1780-1849) realized that calcium, strontium, and barium have similar properties and that the atomic weight of strontium is about halfway between the other two elements. He called this the “law of triads.” He discovered other sets of three elements that followed this law, such as sulfur, selenium, and tellurium, and chlorine, bromine, and iodine. Between 1829 and 1858, several scientists worked on the idea of triads. They discovered that the chemical relationship extended beyond groups of three. Fluorine was added to the halogen group; oxygen, sulfur, selenium, and tellurium were grouped into a family; and nitrogen, phosphorous, arsenic, antimony, and bismuth were grouped into a family. The first comprehensive arrangement of the elements showing the repetition of chemical and physical properties was published in 1862 by French geologist A.E. Beguyer de Chancourtois. -

The Reduction of Elemental Chalcogens and Their Derivatives by Divalent Lanthanide Complexes
Yingzhao Ma (Autor) The Reduction of Elemental Chalcogens and Their Derivatives by Divalent Lanthanide Complexes https://cuvillier.de/de/shop/publications/7834 Copyright: Cuvillier Verlag, Inhaberin Annette Jentzsch-Cuvillier, Nonnenstieg 8, 37075 Göttingen, Germany Telefon: +49 (0)551 54724-0, E-Mail: [email protected], Website: https://cuvillier.de 1 Introduction 1.1 Chalcogen 1.1.1 General The word "chalcogen" is derived from a combination of the Greek word khalkȩs principally meaning copper (the term was also used for bronze/brass, any metal in the poetic sense, ore or coin), and the latinized Greek word genƝs, meaning born or produced.[1] Chalcogens are known as oxygen, sulfur, selenium, tellurium and radioactive element polonium, which are group 16 elements. According to Emsley John, the chemically uncharacterized synthetic element livermorium (Lv) is predicted to be a chalcogen as well.[2] Oxygen was recognized as an element in the 18th century. Sulfur has been known since antiquity as well. Selenium, tellurium and polonium were synthesized in the 19th century, and livermorium in 2000. Almost all the chalcogens can find their roles in biological functions. Typically, lighter chalcogens (oxygen and sulfur) are rarely toxic in their elemental form, and are often critical to life, while the heavier chalcogens (selenium and tellurium) are often toxic.[3] Commercially, selenium is used for glassmaking and pigments. Moreover, selenium is a semiconductor used in photocells electronics. Tellurium is extremely rare in earth’s crust. Compounds baring tellurium were first discovered in Zlatna, Romania by Austrian mineralogist Franz- Joseph Müller von Reichenstein in 1782 in a gold mine, and subsequently, this new element was named by Martin Heinrich Klaproth in 1798. -

Periodic Classification of Elements
CHAPTER 5 Periodic Classification of Elements n Class IX we have learnt that matter around us is present in the form Iof elements, compounds and mixtures and the elements contain atoms of only one type. Do you know how many elements are known till date? At present, 118 elements are known to us. All these have different properties. Out of these 118, only 94 are naturally occurring. As different elements were being discovered, scientists gathered more and more information about the properties of these elements. They found it difficult to organise all that was known about the elements. They started looking for some pattern in their properties, on the basis of which they could study such a large number of elements with ease. 5.1 MAKING ORDER OUT OF CHAOS – EARLYYY AAATTEMPTS AT THE CLASSIFICASSIFICASSIFICAAATION OFOFTION ELEMENTSELEMENTSELEMENTS We have been learning how various things or living beings can be classified on the basis of their properties. Even in other situations, we come across instances of organisation based on some properties. For example, in a shop, soaps are kept together at one place while biscuits are kept together elsewhere. Even among soaps, bathing soaps are stacked separately from washing soaps. Similarly, scientists made several attempts to classify elements according to their properties and obtain an orderly arrangement out of chaos. Figure 5.1 The earliest attempt to classify the elements resulted in Imagine you and your friends have grouping the then known elements as metals and non-metals. found pieces of an old map to reach Later further classifications were tried out as our knowledge a treasure.