Accepted Manuscript
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Status and Protection of Globally Threatened Species in the Caucasus
STATUS AND PROTECTION OF GLOBALLY THREATENED SPECIES IN THE CAUCASUS CEPF Biodiversity Investments in the Caucasus Hotspot 2004-2009 Edited by Nugzar Zazanashvili and David Mallon Tbilisi 2009 The contents of this book do not necessarily reflect the views or policies of CEPF, WWF, or their sponsoring organizations. Neither the CEPF, WWF nor any other entities thereof, assumes any legal liability or responsibility for the accuracy, completeness, or usefulness of any information, product or process disclosed in this book. Citation: Zazanashvili, N. and Mallon, D. (Editors) 2009. Status and Protection of Globally Threatened Species in the Caucasus. Tbilisi: CEPF, WWF. Contour Ltd., 232 pp. ISBN 978-9941-0-2203-6 Design and printing Contour Ltd. 8, Kargareteli st., 0164 Tbilisi, Georgia December 2009 The Critical Ecosystem Partnership Fund (CEPF) is a joint initiative of l’Agence Française de Développement, Conservation International, the Global Environment Facility, the Government of Japan, the MacArthur Foundation and the World Bank. This book shows the effort of the Caucasus NGOs, experts, scientific institutions and governmental agencies for conserving globally threatened species in the Caucasus: CEPF investments in the region made it possible for the first time to carry out simultaneous assessments of species’ populations at national and regional scales, setting up strategies and developing action plans for their survival, as well as implementation of some urgent conservation measures. Contents Foreword 7 Acknowledgments 8 Introduction CEPF Investment in the Caucasus Hotspot A. W. Tordoff, N. Zazanashvili, M. Bitsadze, K. Manvelyan, E. Askerov, V. Krever, S. Kalem, B. Avcioglu, S. Galstyan and R. Mnatsekanov 9 The Caucasus Hotspot N. -

The Treasures of Georgia 10 - 21 September 2022
THE TREASURES OF GEORGIA 10 - 21 SEPTEMBER 2022 FROM £3,395 PER PERSON Tour Leader: Bridget Wheeler RICH HERITAGE, MEDIEVAL HISTORY & NATURAL BEAUTY Situated between the subtropical Black Sea coast, the Caspian Sea and the snowy peaks of the Caucasus, the state of Georgia sits at the crossroads of Europe and Asia. Georgia’s rich heritage has been hugely influenced by both Byzantine and Persian cultures. With Russia (and Chechnya, Ingushetia, Kabardino-Balkar, North Osetia-Alania) to the north, Turkey to the west, Armenia and Iran to the south and Azerbaijan to the east, Georgia remains a fiercely independent nation after years of oppression by the Russian Tsars and the Soviet Union. Georgia’s mix of natural beauty and medieval history has made this small country one of Eastern Europe’s most fascinating destinations. Bodbe Monastery Uplistsikhe Alazani Valley 12-DAY ITINERARY, DEPARTING 10 SEPTEMBER 2022 10 September London ancient land of Colchis, spectacular and filled with historical sights. modern and contemporary paintings Visit Bodbe Nunnery, just outside Suggested flights (not included in the by Georgian artists and masterpieces Sighnaghi. In the 4th century, after cost of the tour) Georgian Airways of Oriental, Western European and Georgia converted to Christianity, A9 752 departing London Gatwick at Russian decorative arts. Dinner at a Saint Nino, the queen responsible for 22.50 hrs. local restaurant. the conversion, withdrew to Bodbe Gorge where she died. She wanted 11 September Tbilisi to be buried in a church in Bodbe 12 September Tbilisi Arrive Tbilisi at 06.35 hrs. Transfer and rather than being taken away to be Morning walking tour of Tbilisi. -

Glaciers Change Over the Last Century, Caucasus Mountains, Georgia
1 Glaciers change over the last century, Caucasus Mountains, 2 Georgia, observed by the old topographical maps, Landsat 3 and ASTER satellite imagery 4 5 L. G. Tielidze 6 7 Department of Geomorphology, Vakhushti Bagrationi Institute of Geography, Ivane 8 Javakhishvili Tbilisi State University, 6 Tamarashvili st. Tbilisi 0177, Georgia 9 10 Correspondence to: L. G. Tielidze ([email protected]) 11 12 13 Abstract 14 15 The study of glaciers in the Caucasus began in the first quarter of the 18th century. The 16 first data on glaciers can be found in the works of great Georgian scientist Vakhushti 17 Bagrationi. After almost hundred years the foreign scientists began to describe the 18 glaciers of Georgia. Information about the glaciers of Georgia can be found in the 19 works of W. Abich, D. Freshfield, G. Radde, N. Dinik, I. Rashevskiy, A. Reinhardt etc. The 20 first statistical information about the glaciers of Georgia are found in the catalog of the 21 Caucasus glaciers compiled by K. Podozerskiy in 1911. Then, in 1960s the large-scale 22 (1 : 25 000, 1 : 50 000) topographic maps were published, which were compiled in 23 1955–1960 on the basis of the airphotos. On the basis of the mentioned maps R. 24 Gobejishvili gave quite detailed statistical information about the glaciers of Georgia. Then 25 in 1975 the results of glaciers inventory of the former USSR was published, where the 26 statistical information about the glaciers of Georgia was obtained on the basis of the 27 almost same time (1955-1957) aerial images. -

Wikivoyage Georgia.Pdf
WikiVoyage Georgia March 2016 Contents 1 Georgia (country) 1 1.1 Regions ................................................ 1 1.2 Cities ................................................. 1 1.3 Other destinations ........................................... 1 1.4 Understand .............................................. 2 1.4.1 People ............................................. 3 1.5 Get in ................................................. 3 1.5.1 Visas ............................................. 3 1.5.2 By plane ............................................ 4 1.5.3 By bus ............................................. 4 1.5.4 By minibus .......................................... 4 1.5.5 By car ............................................. 4 1.5.6 By train ............................................ 5 1.5.7 By boat ............................................ 5 1.6 Get around ............................................... 5 1.6.1 Taxi .............................................. 5 1.6.2 Minibus ............................................ 5 1.6.3 By train ............................................ 5 1.6.4 By bike ............................................ 5 1.6.5 City Bus ............................................ 5 1.6.6 Mountain Travel ....................................... 6 1.7 Talk .................................................. 6 1.8 See ................................................... 6 1.9 Do ................................................... 7 1.10 Buy .................................................. 7 1.10.1 -

Status and Protection of Globally Threatened Species in the Caucasus
STATUS AND PROTECTION OF GLOBALLY THREATENED SPECIES IN THE CAUCASUS CEPF Biodiversity Investments in the Caucasus Hotspot 2004-2009 Edited by Nugzar Zazanashvili and David Mallon Tbilisi 2009 The contents of this book do not necessarily re ect the views or policies of CEPF, WWF, or their sponsoring organizations. Neither the CEPF, WWF nor any other entities thereof, assumes any legal liability or responsibility for the accuracy, completeness, or usefulness of any information, product or process disclosed in this book. Citation: Zazanashvili, N. and Mallon, D. (Editors) 2009. Status and Protection of Globally Threatened Species in the Caucasus. Tbilisi: CEPF, WWF. Contour Ltd., 232 pp. ISBN 978-9941-0-2203-6 Design and printing Contour Ltd. 8, Kargareteli st., 0164 Tbilisi, Georgia December 2009 The Critical Ecosystem Partnership Fund (CEPF) is a joint initiative of l’Agence Française de Développement, Conservation International, the Global Environment Facility, the Government of Japan, the MacArthur Foundation and the World Bank. This book shows the effort of the Caucasus NGOs, experts, scienti c institutions and governmental agencies for conserving globally threatened species in the Caucasus: CEPF investments in the region made it possible for the rst time to carry out simultaneous assessments of species’ populations at national and regional scales, setting up strategies and developing action plans for their survival, as well as implementation of some urgent conservation measures. Contents Foreword 7 Acknowledgments 8 Introduction CEPF Investment in the Caucasus Hotspot A. W. Tordoff, N. Zazanashvili, M. Bitsadze, K. Manvelyan, E. Askerov, V. Krever, S. Kalem, B. Avcioglu, S. Galstyan and R. Mnatsekanov 9 The Caucasus Hotspot N. -
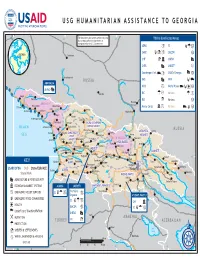
Georgia Program Maps 10/31/2008
USG HUMANITARIAN ASSISTANCE TO GEORGIA 40° E 42° E The boundaries and names used on this map 44° E T'bilisi & Affected46° E Areas Majkop do not imply official endorsement or acceptance by the U.S. Government. ADRA a SC Ga I GEORGIA CARE a UMCOR a Cherkessk CHF IC UNFAO CaspianA Sea 44° CNFA A UNICEF J N 44° Kuban' Counterpart Int. Ea USAID/Georgia Aa N Karachayevsk RUSSIA FAO A WFP E ABKHAZIA E !0 Psou IOCC a World Vision Da !0 UNFAO A 0 Nal'chik IRC G J Various G a ! Gagra Bzyb' Groznyy RUSSIA 0 Pskhu IRD I Various a ! Nazran "ABKHAZIA" Novvy Afon Pitsunda 0 Omarishara Mercy Corps Ca Various E a ! Lata Sukhumi Mestia Gudauta!0 !0 Kodori Inguri Vladikavkaz Otap !0 Khaishi Kvemo-gulripsh Lentekhi !0 Tkvarcheli Dzhvari RACHA-LECHKHUMI-RACHA-LECHKHUMI- Terek BLACK Ochamchira Gali Tsalenjhikha KVEMOKVEMO SVANETISVANETI RUSSIA Khvanchkara Rioni MTSKHETA-MTSKHETA- Achilo Pichori Zugdidi SAMEGRELO-SAMEGRELO- Kvaisi Mleta SEA ZEMOZEMO Ambrolauri MTIANETIMTIANETI Pasanauri Alazani Khobi Tskhaltubo Tkibuli "SOUTH OSSETIA" Anaklia SVANETISVANETI Aragvi Qvirila SHIDASHIDA KARTLIKARTLI Senaki Kurta Artani Rioni Samtredia Kutaisi Chiatura Tskhinvali Poti IMERETIIMERETI Lanchkhuti Rioni !0 Akhalgori KAKHETIKAKHETI Chokhatauri Zestafoni Khashuri N Supsa Baghdati Dusheti N 42° Kareli Akhmeta Kvareli 42° Ozurgeti Gori Kaspi Borzhomi Lagodekhi KEY Kobuleti GURIAGURIA Bakhmaro Borjomi TBILISITBILISI Telavi Abastumani Mtskheta Gurdzhaani Belokany USAID/OFDA DoD State/EUR/ACE Atskuri T'bilisi Î! Batumi 0 AJARIAAJARIA Iori ! Vale Akhaltsikhe Zakataly State/PRM -

Ancient Glaciation of the Caucasus
Open Journal of Geology, 2018, 8, 56-64 http://www.scirp.org/journal/ojg ISSN Online: 2161-7589 ISSN Print: 2161-7570 Ancient Glaciation of the Caucasus Khazaradze Revaz, Kharadze Koba, Tsikarishvili Kukuri, Chartolani Giorgi Vakhushti Bagrationi Institute of Geography, Ivane Javakhishvili Tbilisi State University, Tbilisi, Georgia How to cite this paper: Revaz, K., Koba, Abstract K., Kukuri, T. and Giorgi, C. (2018) An- cient Glaciation of the Caucasus. Open The paper deals with important, different views on the old glaciation of the Journal of Geology, 8, 56-64. Caucasus based on the literary sources and the latest data obtained through https://doi.org/10.4236/ojg.2018.81004 the expeditions. Our researches are mostly of complex character where petro- Received: December 8, 2017 graphic, palynological and paleontological methods are widely used. The pe- Accepted: January 20, 2018 trographic method allowed us to connect block debris of the moraine with the Published: January 23, 2018 main centers and to determine the directions of the glaciers movement and Copyright © 2018 by authors and their distribution boundaries. The palynological method was used to deter- Scientific Research Publishing Inc. mine the genesis of loose sediments considered as moraines and their sedi- This work is licensed under the Creative mentation conditions. The palynological spectrum of deposits shows that they Commons Attribution International License (CC BY 4.0). are river sediments and their sedimentation took place under humid, mod- http://creativecommons.org/licenses/by/4.0/ erately warm climate conditions that is not associated with glaciation. Open Access Keywords Petrography, Palynology, Genesis, Sedimentation 1. Introduction The study of the Caucasian Isthmus, from the point of view of paleoglaciation, began in the second part of the XIX century [1]. -

Genesis and Sedimentation of the Travertines in Georgia
saqarTvelos mecnierebaTa erovnuli akademiis moambe, t. 14, #1, 2020 BULLETIN OF THE GEORGIAN NATIONAL ACADEMY OF SCIENCES, vol. 14, no. 1, 2020 Paleogeography Genesis and Sedimentation of the Travertines in Georgia Revaz Khazaradze* and Koba Kharadze* Vakhushti Bagrationi Institute of Geography, Ivane Javakhishvili Tbilisi State University, Tbilisi, Georgia (Presented by Academy Member Revaz Gachechiladze) On the basis of the studies carried out we determined the areas of the genesis and distribution of travertines on the territory of Georgia. As it became clear the distribution of travertines in the Caucasus Mountains is mainly linked to the distribution line of Mesozoic sediments, where two types of travertines can be distinguished: an active travertine (it has a source and sedimentation is still lasting) and an inactive travertine (the source has dried up and sedimentation does not take place). In the active travertines there are numerous prints of plant leaves and stems that enables to determine the approximate age of their genesis; it became known that it may belong to Holocene or a period close to the modern age. As to the inactive travertines, they are poor in vegetation prints. They have been eroded and therefore, it becomes difficult to determine their age. © 2020 Bull. Georg. Natl. Acad. Sci. Travertines, active travertine, inactive travertine, genesis Water saturated with the carbonate material following the glaciation (Holocene). Seemingly, deposits it onto layer surfaces in the forms of the above researchers share the idea that mineral limestone, tufa and travertine. The outcropped waters were widely spread in the Quaternary in the rocks are not always presented as limestone. -

Pdf | 281.89 Kb
USG HUMANITARIAN ASSISTANCE TO GEORGIA 40° E 42° E 44° E The46° boundariesE and names used on this map Majkop do not imply official endorsement or acceptance by the U.S. Government. Caspian GEORGIA Cherkessk Sea N N 44° 44° Kuban' T'bilisi & Affected Areas Karachayevsk RUSSIA CARE a Psou E !0 !0 Counterpart Int. a 0 Nal'chik FAO A ! Gagra Bzyb' Groznyy !0 Pskhu UMCOR a Novvy Afon Nazran Pitsunda !0 "ABKHAZIA" Omarishara UNICEF J Lata Mestia Gudauta!0 0 Sukhumi Kodori Inguri WFP ! Vladikavkaz Otap USAID/GeorgiaRUSSIA a !0 Khaishi Kvemo-gulripsh Lentekhi WFP a EF !0 Tkvarcheli Dzhvari RACHA-LECHKHUMI- Terek KVEMO SVANETI Ochamchira Gali Tsalenjhikha World Vision a Khvanchkara Rioni MTSKHETA- Achilo Pichori Zugdidi SAMEGRELO- Kvaisi Mleta ZEMO Ambrolauri MTIANETI Various a Pasanauri Alazani Khobi Tskhaltubo Tkibuli "SOUTH OSSETIA" Anaklia SVANETI Aragvi Qvirila SHIDA KARTLI Various E a BLACK Senaki Kurta Artani Rioni Samtredia Kutaisi Chiatura Tskhinvali Poti IMERETI Lanchkhuti Rioni !0 Akhalgori KAKHETI RUSSIA SEA Chokhatauri Zestafoni Khashuri N Supsa Baghdati Dusheti N 42° Kareli Akhmeta Kvareli 42° Ozurgeti Gori Kaspi Borzhomi Lagodekhi Kobuleti GURIA Bakhmaro Borjomi TBILISI Telavi Abastumani Mtskheta Gurdzhaani Belokany Atskuri T'bilisi Î! Batumi 0 AJARIA Iori ! Vale Akhaltsikhe Zakataly Khulo Rustavi Keda KVEMO KARTLI Tsnori Bolnisi !0 Akhalkalaki Marneuli Dedoplis Tsqaro SAMTSKHE-JAVAKHETI Dmanisi KEY Iori Coruh Ninotsminda Kura USAID/OFDA DoD State/EUR/ACE Mingachever !0 !0 Resevoir A AGRICULTURE AND FOOD SECURITY Rize a EMERGENCY RELIEF SUPPLIES TURKEY ARMENIA Kars EMERGENCY FOOD COMMODITIES AZERBAIJAN E LOGISTICS & TRANSPORTATION Arteni Lake INFORM NUTRITION IC AT F Sevan PH IO A N Î! R G U N O Yerevan E I WATER, SANITATION & HYGIENE T J 0 30 60 mi G Firat Aras N U N 8/27/08 S A 40° A 40°D 0 30 60 90 km I F D O 40° E 42° E 44° E 46° E / D C H A /. -

This Learning Toolkit Was Developed in the Framework of the UNDP-GEF
1 This learning toolkit was developed in the framework of the UNDP-GEF project “Advancing Integrated Water Resource Management (IWRM) across the Kura river basin through implementation of the transboundary agreed actions and national plans”. It aims to provide a better understanding of the current state of water resources in the Kura river basin. It examines links between human activities and environmental degradation in the basin, as well as potential impacts of such global threats as climate change and disasters on water resources of the Kura river basin. The publication also includes interesting facts about water resources, related ecosystems and provides additional information about some environmental concepts. The toolkit is applicable as an additional source of information for the schoolteachers, students and everyone else who uses water. Contributors: Mary Matthews, Nino Malashkhia, Hajar Huseynova, Ahmed Abou Elseoud, Tamar Gugushvili, Elchin Mammadov, Jeanene Mitchell, Surkhay Shukurov, Aysel Muradova, Maia Ochigava, Sona Guliyeva Disclaimer: The views expressed in this publication are those of the UNDP-GEF Kura II Project Team and do not necessarily represent those of the United Nations or UNDP or the Global Environment Facility TABLE OF CONTENTS 1. Introduction ....................................................................................................9 Is water an economic or social good? ..................................................................13 2. Water cycle in a nutshell .............................................................................15 -

Causes of War Prospects for Peace
Georgian Orthodox Church Konrad-Adenauer-Stiftung CAUSES OF WAR PROS P E C TS FOR PEA C E Tbilisi, 2009 1 On December 2-3, 2008 the Holy Synod of the Georgian Orthodox Church and the Konrad-Adenauer-Stiftung held a scientific conference on the theme: Causes of War - Prospects for Peace. The main purpose of the conference was to show the essence of the existing conflicts in Georgia and to prepare objective scientific and information basis. This book is a collection of conference reports and discussion materials that on the request of the editorial board has been presented in article format. Publishers: Metropolitan Ananya Japaridze Katia Christina Plate Bidzina Lebanidze Nato Asatiani Editorial board: Archimandrite Adam (Akhaladze), Tamaz Beradze, Rozeta Gujejiani, Roland Topchishvili, Mariam Lordkipanidze, Lela Margiani, Tariel Putkaradze, Bezhan Khorava Reviewers: Zurab Tvalchrelidze Revaz Sherozia Giorgi Cheishvili Otar Janelidze Editorial board wishes to acknowledge the assistance of Irina Bibileishvili, Merab Gvazava, Nia Gogokhia, Ekaterine Dadiani, Zviad Kvilitaia, Giorgi Cheishvili, Kakhaber Tsulaia. ISBN 2345632456 Printed by CGS ltd 2 Preface by His Holiness and Beatitude Catholicos-Patriarch of All Georgia ILIA II; Opening Words to the Conference 5 Preface by Katja Christina Plate, Head of the Regional Office for Political Dialogue in the South Caucasus of the Konrad-Adenauer-Stiftung; Opening Words to the Conference 8 Abkhazia: Historical-Political and Ethnic Processes Tamaz Beradze, Konstantine Topuria, Bezhan Khorava - A -

2 Goldstein Georgian Feast.Indd 1 28/07/18 6:22 PM 1 a TASTE of HISTORY
PART I AN ABUNDANT LAND michael kenna michael © 2_Goldstein_Georgian Feast.indd 1 28/07/18 6:22 PM 1 A TASTE OF HISTORY Visitors from the kolkhoz celebrating Saint George’s Day at the Alaverdi Monastery near Telavi, 1972. The eleventh-century Alaverdi Monastery was for centuries the tallest church in Georgia. The holiday of Saint George, one of Georgia’s patron saints, is celebrated twice a year, on November 23 and May 6. © Magnum photos / Henri Cartier-Bresson (previous page) Ruins of an old church at Ananuri Fortress, 2008. The village of Ananuri, about an hour north of Tbilisi, once lay along the fabled Silk Road. The dukes of Aragvi built the fortress to protect their domain, which they ruled from the thirteenth to eighteenth centuries. © Michael Kenna 2_Goldstein_Georgian Feast.indd 2 28/07/18 6:22 PM remote as georgia may seem, this small nation once occupied a pivotal place in the world. Georgia was much featured in the mythology of ancient times, chroni- cled by historians and travelers who ventured to the farthest reaches of the known classical world. Stretching from the Black Sea to the Caspian, Georgia lay athwart important East–West trade routes. Through the vagaries of history, Georgia enjoyed independence one thousand years ago, only to be subsumed by the Russian Empire in the nineteenth century. More recently, the republic existed within the borders of the Soviet Union. In 1991, the Soviets’ relaxed hold on their constituent republics allowed the proud and restive Georgians to proclaim independence. With a num- ber of contending political groups, Georgia today fi nds itself in fl ux.