`Degraded' RNA Profiles in Arthropoda and Beyond
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Marine Invertebrate Field Guide
Marine Invertebrate Field Guide Contents ANEMONES ....................................................................................................................................................................................... 2 AGGREGATING ANEMONE (ANTHOPLEURA ELEGANTISSIMA) ............................................................................................................................... 2 BROODING ANEMONE (EPIACTIS PROLIFERA) ................................................................................................................................................... 2 CHRISTMAS ANEMONE (URTICINA CRASSICORNIS) ............................................................................................................................................ 3 PLUMOSE ANEMONE (METRIDIUM SENILE) ..................................................................................................................................................... 3 BARNACLES ....................................................................................................................................................................................... 4 ACORN BARNACLE (BALANUS GLANDULA) ....................................................................................................................................................... 4 HAYSTACK BARNACLE (SEMIBALANUS CARIOSUS) .............................................................................................................................................. 4 CHITONS ........................................................................................................................................................................................... -
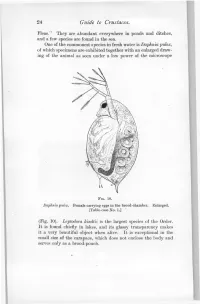
24 Guide to Crustacea
24 Guide to Crustacea. Fleas." They are abundant everywhere in ponds and ditches, and a few species are found in the sea. One of the commonest species in fresh water is Daphnia pulex, of which specimens are exhibited together with an enlarged draw- ing of the animal as seen under a low power of the microscope FIG. 10. Daphnia pulex. Female carrying eggs in the brood-chamber. Enlarged. [Table-case No. 1.] (Fig. 10). Leptodora kindtii is the largest species of the Order. It is found chiefly in lakes, and its glassy transparency makes it a very beautiful object when alive. It is exceptional in the small size of the carapace, which does not enclose the body and serves only as a brood-pouch. Ostracoda. 25 Sub-class II.—OSTRACODA. (Table-ease No. 1.) The number of somites, as indicated by the appendages, is smaller than in any other Crustacea, there being, at most, only two pairs of trunk-limbs behind those belonging to the head- region. The carapace forms a bivalved shell completely en- closing the body and limbs. There is a large, and often leg-like, palp on the mandible. The antennules and antennae are used for creeping or swimming. The Ostracoda (Fig. 10) are for the most part extremely minute animals, and only one or two of the larger species can be exhibited. They occur abundantly in fresh water and in FIG. 11. Shells of Ostracoda, seen from the side. A. Philomedes brenda (Myodocopa) ; B. Cypris fuscata (Podocopa); ('. Cythereis ornata (Podocopa): all much enlarged, n., Notch characteristic of the Myodocopa; e., the median eye ; a., mark of attachment of the muscle connecting the two valves of the shell. -

Dosima Fascicularis (Ellis & Solander, 1786) (Crustacea, Cirripedia) from the Galician Istributio D
Check List 10(3): 669–671, 2014 © 2014 Check List and Authors Chec List ISSN 1809-127X (available at www.checklist.org.br) Journal of species lists and distribution N First record of the buoy barnacle Dosima fascicularis (Ellis & Solander, 1786) (Crustacea, Cirripedia) from the Galician ISTRIBUTIO D * RAPHIC G beaches (NW Spain) after the Prestige oil spill EO G Juan Junoy and Jorge Junoy N O EU-US Marine Biodiversity [email protected] Group, Franklin Institute and Departamento de Ciencias de la Vida, AP 20. Campus Universitario, Universidad de Alcalá, 28805 Alcalá de Henares, Spain. OTES * Corresponding author. E-mail: N Abstract: Dosima fascicularis The presence of the buoy barnacle is first documented for Spanish waters. More than the half of the specimens collected was attached to tar pellets from the Prestige oil spill. DOI: 10.15560/10.3.669 Dosima fascicularis (Ellis & Solander, 1786) is a presumablycollected along from the the 1470 Prestige m of oil the spill; beach other longitude. attachment The stalked barnacle which capitulum bears five large plates. surfacesfloats of 53.8%were Velellaof the specimens were Sargassumattached to bladderstar balls, It is the unique barnacle that constructs its own float, a attachmentfoamy mass surfaces described (Darwin as similar 1851; to Minchin polystyrene, 1996; usingRyan tests (15.3%), floating items as feathers, tar balls and plastic particles as (7.6%) and feathers (2.5%). 20.8% of buoy barnacles lacked an obvious attachment object (Figure 2). theand BritishBranch Islands2012). The and species Baltic Seais widespread (Darwin 1851) in temperate but its sizeThe recorded average for thecapitulum species lengthwas 37 ofmm the in Europeancollected presenceand subtropical in European ocean waters,coast is reaching unusual in(O’ the Riodan NE Atlantic 1967; specimens was 17.7 mm (range 13–22 mm). -

Using Growth Rates to Estimate Age of the Sea Turtle Barnacle Chelonibia Testudinaria
Mar Biol (2017) 164:222 DOI 10.1007/s00227-017-3251-5 SHORT NOTE Using growth rates to estimate age of the sea turtle barnacle Chelonibia testudinaria Sophie A. Doell1 · Rod M. Connolly1 · Colin J. Limpus2 · Ryan M. Pearson1 · Jason P. van de Merwe1 Received: 4 May 2017 / Accepted: 20 October 2017 © Springer-Verlag GmbH Germany 2017 Abstract Epibionts can serve as valuable ecological indi- may live for up to 2 years, means that these barnacles may cators, providing information about the behaviour or health serve as interesting ecological indicators over this period. of the host. The use of epibionts as indicators is, however, In turn, this information may be used to better understand often limited by a lack of knowledge about the basic ecology the movement and habitat use of their sea turtle hosts, ulti- of these ‘hitchhikers’. This study investigated the growth mately improving conservation and management of these rates of a turtle barnacle, Chelonibia testudinaria, under threatened animals. natural conditions, and then used the resulting growth curve to estimate the barnacle’s age. Repeat morphomet- ric measurements (length and basal area) on 78 barnacles Introduction were taken, as host loggerhead turtles (Caretta caretta) laid successive clutches at Mon Repos, Australia, during the Sea turtles are known to host diverse communities of plants 2015/16 nesting season. Barnacles when frst encountered and invertebrate animals (Caine 1986; Kitsos et al. 2005; ranged in size from 3.7 to 62.9 mm, and were recaptured Robinson et al. 2017). Past analyses of the size, abundance, between 12 and 56 days later. -

Chilean Rose-Haired Tarantula Native Range Map
Chilean Rose-haired Tarantula Native Range Map Kingdom: Animalia Phylum: Arthropoda Subphylum: Chelicerata Class: Arachnida Order: Araneae Family: Theraphosidae Genus : Grammostola Species : gala Photo courtesy of Karen Marzynski Habitat • In the Wild: This species of tarantula can be found in Chile, in dry grassland regions at the edge of the desert. • Exhibit Location: Zoo to You Collection Characteristics • Adults grow to be 4.5 – 5.5 inches in diameter. • There are 2 different color schemes, depending on where in Chile they are from. Many are brownish, while others are more reddish or pink in color. • This tarantula has a hard external skeleton (exoskeleton) and 8 jointed legs. The exterior of the body is covered by long, bristle-like hairs. There is a smaller pair of sensory appendages called pedipalps. They have 8 eyes, 2 fangs, and are venomous (poisonous). They have a cephalothorax (composed of the head and thorax) to which all appendages except the spinnerets (tubular structures from which web silk are produced) are attached. The spinnerets are found on the abdomen. • Individual hairs may be sensitive to motion, heat, cold, and other environmental triggers. Hairs near the mouth are capable of sensing chemicals that give the spider a basic type of sense of smell and taste. • Lifespan: In the Wild males 3-10 years, females 15-20 years; In Captivity males less than 2 years, females 20 or more years (average is 12 years) Behaviors • The Chilean rose-haired tarantula is a nocturnal (nighttime) hunter and finds a shelter to web itself into at dawn. • Their digestive system is designed to deal with liquid food only. -

A New Species of Lepas (Crustacea: Cirripedia: Pedunculata) from the Miocene Mizunami Group, Japan
Bulletin of the Mizunami Fossil Museum, no. 31 (2004), p. 91-93, 1 fig. c 2004, Mizunami Fossil Museum A new species of Lepas (Crustacea: Cirripedia: Pedunculata) from the Miocene Mizunami Group, Japan Hiroaki Karasawa*, Toshio Tanaka* *, and Yoshitsugu Okumura*** * Mizunami Fossil Museum, Yamanouchi, Akeyo, Mizunami, Gifu 509-6132, Japan <[email protected]> ** Aichi Gakuin Junior College, Chikusa, Nagoya 464-8650, Japan < [email protected]> *** Mizunami Fossil Museum, Yamanouchi, Akeyo, Mizunami, Gifu 509-6132, Japan <[email protected]> Abstract Lepas kuwayamai, a new species of a lepadid thracican is described from the lower Miocene Mizunami Group in Gifu Prefecture, Honshu, Japan. This represents the third record for the family from Miocene deposits of Japan. Key words: Crustacea, Cirripedia, Thoracica, Pedunculata, Lepadidae, Lepas, Miocene, Japan Material examined: MFM9043, holotype; 8 paratypes, Introduction MFM9044-9051; Coll. M. Kuwayama in, 2004. All specimens are housed in the Mizunami Fossil Museum. The lepadomorph family Lepadidae Darwin, 1851, is a Diagnosis: Lepas with moderate sized capitulum. Shell small group including six genera (Newman, 1996). Among thick. Scutum triangular, slightly higher than wide, with these, the genus Lepas Linnaeus, 1758, is only known clear growth lines; umbonal tooth absent; very weak from Japan in the fossil record. O’hara et al. (1976) reported radial striae sometimes present; apicoumbonal ridge Lepas sp. from the middle Pleistocene Shimosa Group and weak. Tergum flattened without radial striae; occludent unnamed Miocene species was reported from the lower margin convex, rounded. Carina broad. Miocene Morozaki Group (Mizuno and Takeda, 1993) and Etymology: In honor to our friend, Mr. -

Occurrence of Sea Spider Endeis Mollis Carpenter (Arthropoda: Pycnogonida) on the Test Panels Submerged in Gulf of Mannar, Southeast Coast of India
Arthropods, 2012, 1(2):73-78 Article Occurrence of sea spider Endeis mollis Carpenter (Arthropoda: Pycnogonida) on the test panels submerged in Gulf of Mannar, southeast coast of India S. Satheesh*, S. G. Wesley Department of Zoology, Scott Christian College (Autonomous), Nagercoil-629003, Tamil Nadu, India *Present address: Department of Marine Biology, Faculty of Marine Sciences, King Abdulaziz University, Post Box No. 80207, Jeddah-21589, Saudi Arabia E-mail: [email protected] Received 27 February 2012; Accepted 1 April 2012; Published online 5 June 2012 IAEES Abstract Sea spiders (Pycnogonids) are exclusively marine arthropods with worldwide distribution. Pycnogonida remains one of the poorly investigated groups encountered in fouling communities. In the present study, distribution pycnogonid species Endeis mollis associated with the fouling community developed on test panels submerged at Kudankulam coast, Gulf of Mannar was studied for a period of two years. Throughout the period of investigation, Endeis mollis was observed on the test panels. A maximum of 55 individuals per square dm was observed during pre-monsoon season and a minimum of 9 individuals per square dm during monsoon season. Results of this study on seasonal distribution are of considerable interest because so little has been documented on the ecology of Pycnogonids in India. Keywords arthropods; biofouling; fouling community; Pycnogonids; Kudankulam; Endeis mollis; biogeography. 1 Introduction Sea spiders or pycnogonids are distinct group of arthropods, exclusively found in the intertidal to abyssal depths in all the marine waters of the world (Arango, 2003; Carranza et al., 2007; Fornshell, 2012). They are commonly found as epibenthic community on other invertebrates, algae, corals etc (Child and Harbison, 1986). -

TARANTULA Araneae Family: Theraphosidae Genus: 113 Genera
TARANTULA Araneae Family: Theraphosidae Genus: 113 genera Range: World wide Habitat tropical and desert regions; greatest concentration S America Niche: Terrestrial or arboreal, carnivorous, mainly nocturnal predators Wild diet: as grasshoppers, crickets and beetles but some of the larger species may also eat mice, lizards and frogs or even small birds Zoo diet: Life Span: (Wild) varies with species and sexes, females tend to live long lives (Captivity) Sexual dimorphism: Location in SF Zoo: Children’s Zoo - Insect Zoo APPEARANCE & PHYSICAL ADAPTATIONS: Tarantulas are large, long-legged, long-living spiders, whose entire body is covered with short hairs, which are sensitive to vibration. They have eight simple eyes arranged in two distinct rows but rely on their hairs to send messages of local movement. These spiders do not spin a web but catch their prey by pursuit, killing them by injecting venom through their fangs. The injected venom liquefies their prey, allowing them to suck out the innards and leave the empty exoskeleton. The chelicerae are vertical and point downward making it necessary to raise its front end to strike forward and down onto its prey. Tarantulas have two pair of book lungs, which are situated on the underside of the abdomen. (Most spiders have only one pair). All tarantulas produce silk through the two or four spinnerets at the end of their abdomen (A typical spiders averages six). New World Tarantulas vs. Old World Tarantulas: New World species have urticating hairs that causes the potential predator to itch and be distracted so the tarantula can get away. They are less aggressive than Old World Tarantulas who lack urticating hairs and their venom is less potent. -

Battle of the Barnacles
Gary Skinner Battle of the barnacles Competition on the seashore The photograph on the centre pages shows a themselves, head down, on suitable rocks, build a rock wall on a British rocky shore. It is covered shell, poke their legs out of the top of it and start to filter feed! Barnacles like this are called acorn with various intertidal animals, mainly barnacles, and they occur in mind boggling numbers barnacles. Have a good look at the image; you on most rocky beaches around the world. should be able to see barnacles of two different species and, amongst them, and even sometimes in their old shells, other animals, mainly shelled ones called molluscs. This image can be used to go on a virtual tour of this part of the rocky shore, and to see – nearly first hand – some important principles of ecology. uch of the UK coastline is rocky, and the parts washed by the sea from high Children might be doing worse at school then they otherwise would to low tide are called rocky shores. The M © Christoph Corteau/naturepl.com region between the tides is the littoral zone, home because they can’t pay attention in class after eating the colourings. A British barnacle, Semibalanus balanoides, to many species of animals and algae (but no true reveals its legs when the tide comes in. plants). A day spent searching for living creatures on a rocky shore, it is claimed, can yield creatures from Box 1 over twenty different phyla (singular phylum; see What is a phylum? Box 1). -

Araneae (Spider) Photos
Araneae (Spider) Photos Araneae (Spiders) About Information on: Spider Photos of Links to WWW Spiders Spiders of North America Relationships Spider Groups Spider Resources -- An Identification Manual About Spiders As in the other arachnid orders, appendage specialization is very important in the evolution of spiders. In spiders the five pairs of appendages of the prosoma (one of the two main body sections) that follow the chelicerae are the pedipalps followed by four pairs of walking legs. The pedipalps are modified to serve as mating organs by mature male spiders. These modifications are often very complicated and differences in their structure are important characteristics used by araneologists in the classification of spiders. Pedipalps in female spiders are structurally much simpler and are used for sensing, manipulating food and sometimes in locomotion. It is relatively easy to tell mature or nearly mature males from female spiders (at least in most groups) by looking at the pedipalps -- in females they look like functional but small legs while in males the ends tend to be enlarged, often greatly so. In young spiders these differences are not evident. There are also appendages on the opisthosoma (the rear body section, the one with no walking legs) the best known being the spinnerets. In the first spiders there were four pairs of spinnerets. Living spiders may have four e.g., (liphistiomorph spiders) or three pairs (e.g., mygalomorph and ecribellate araneomorphs) or three paris of spinnerets and a silk spinning plate called a cribellum (the earliest and many extant araneomorph spiders). Spinnerets' history as appendages is suggested in part by their being projections away from the opisthosoma and the fact that they may retain muscles for movement Much of the success of spiders traces directly to their extensive use of silk and poison. -

Title FEEDING PREFERENCES and RATES of the SNAIL, IANTHINA
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Kyoto University Research Information Repository FEEDING PREFERENCES AND RATES OF THE SNAIL, IANTHINA PROLONGATA, THE BARNACLE, LEPAS Title ANSERIFERA, THE NUDIBRANCHS, GLAUCUS ATLANTICUS AND FIONA PINNATA, AND THE FOOD WEB IN THE MARINE NEUSTON Author(s) Bieri, Robert PUBLICATIONS OF THE SETO MARINE BIOLOGICAL Citation LABORATORY (1966), 14(2): 161-170 Issue Date 1966-06-30 URL http://hdl.handle.net/2433/175429 Right Type Departmental Bulletin Paper Textversion publisher Kyoto University FEEDING PREFERENCES AND RATES OF THE SNAIL, IANTHINA PROLONGATA, THE BARNACLE, LEPAS ANSERIFERA, THE NUDIBRANCHS, GLAUCUS ATLANTICUS AND FIONA PINNATA, AND THE FOOD WEB IN THE MARINE NEUSTON'l RoBERT BIERI Antioch College, Yellow Springs, Ohio With Plates III-IV and 1 Text-figure On the afternoon of November 5, 1965 during strong north-northwest winds, Porpita, Velella, Physalia, G!aucus, Lepas, Fiona, and three species of Ianthina were blown onto the north beach of the Seto Marine Biological Laboratory. In the Laboratory with an ample supply of running sea water, many of these animals lived for several days and I was able to observe their feeding preferences and rates. On the basis of these obervations and the reports of other workers it is possible to construct a preliminary diagram of the neuston food web. Ianthina prolongata Of the three species of Ianthina washed ashore, I. janthina forma balteata, I. umbilicata, and I. prolongata, I. prolongata was most abundant. I was able to collect more than 160 of them in less than an hour. -

UNIVERSITY of CALIFORNIA, SAN DIEGO Abundance and Ecological
UNIVERSITY OF CALIFORNIA, SAN DIEGO Abundance and ecological implications of microplastic debris in the North Pacific Subtropical Gyre A dissertation submitted in partial satisfaction of the requirements for the degree Doctor of Philosophy in Oceanography by Miriam Chanita Goldstein Committee in charge: Professor Mark D. Ohman, Chair Professor Lihini I. Aluwihare Professor Brian Goldfarb Professor Michael R. Landry Professor James J. Leichter 2012 Copyright Miriam Chanita Goldstein, 2012 All rights reserved. SIGNATURE PAGE The Dissertation of Miriam Chanita Goldstein is approved, and it is acceptable in quality and form for publication on microfilm and electronically: PAGE _____________________________________________________________________ _____________________________________________________________________ _____________________________________________________________________ _____________________________________________________________________ _____________________________________________________________________ Chair University of California, San Diego 2012 iii DEDICATION For my mother, who took me to the tidepools and didn’t mind my pet earthworms. iv TABLE OF CONTENTS SIGNATURE PAGE ................................................................................................... iii DEDICATION ............................................................................................................. iv TABLE OF CONTENTS ............................................................................................. v LIST OF FIGURES