The Genus Hippolyte Leach, 1814
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

DLA Piper. Details of the Member Entities of DLA Piper Are Available on the Website
EUROPEAN PPP REPORT 2009 ACKNOWLEDGEMENTS This Report has been published with particular thanks to: The EPEC Executive and in particular, Livia Dumitrescu, Goetz von Thadden, Mathieu Nemoz and Laura Potten. Those EPEC Members and EIB staff who commented on the country reports. Each of the contributors of a ‘View from a Country’. Line Markert and Mikkel Fritsch from Horten for assistance with the report on Denmark. Andrei Aganimov from Borenius & Kemppinen for assistance with the report on Finland. Maura Capoulas Santos and Alberto Galhardo Simões from Miranda Correia Amendoeira & Associados for assistance with the report on Portugal. Gustaf Reuterskiöld and Malin Cope from DLA Nordic for assistance with the report on Sweden. Infra-News for assistance generally and in particular with the project lists. All those members of DLA Piper who assisted with the preparation of the country reports and finally, Rosemary Bointon, Editor of the Report. Production of Report and Copyright This European PPP Report 2009 ( “Report”) has been produced and edited by DLA Piper*. DLA Piper acknowledges the contribution of the European PPP Expertise Centre (EPEC)** in the preparation of the Report. DLA Piper retains editorial responsibility for the Report. In contributing to the Report neither the European Investment Bank, EPEC, EPEC’s Members, nor any Contributor*** indicates or implies agreement with, or endorsement of, any part of the Report. This document is the copyright of DLA Piper and the Contributors. This document is confidential and personal to you. It is provided to you on the understanding that it is not to be re-used in any way, duplicated or distributed without the written consent of DLA Piper or the relevant Contributor. -

Dosima Fascicularis (Ellis & Solander, 1786) (Crustacea, Cirripedia) from the Galician Istributio D
Check List 10(3): 669–671, 2014 © 2014 Check List and Authors Chec List ISSN 1809-127X (available at www.checklist.org.br) Journal of species lists and distribution N First record of the buoy barnacle Dosima fascicularis (Ellis & Solander, 1786) (Crustacea, Cirripedia) from the Galician ISTRIBUTIO D * RAPHIC G beaches (NW Spain) after the Prestige oil spill EO G Juan Junoy and Jorge Junoy N O EU-US Marine Biodiversity [email protected] Group, Franklin Institute and Departamento de Ciencias de la Vida, AP 20. Campus Universitario, Universidad de Alcalá, 28805 Alcalá de Henares, Spain. OTES * Corresponding author. E-mail: N Abstract: Dosima fascicularis The presence of the buoy barnacle is first documented for Spanish waters. More than the half of the specimens collected was attached to tar pellets from the Prestige oil spill. DOI: 10.15560/10.3.669 Dosima fascicularis (Ellis & Solander, 1786) is a presumablycollected along from the the 1470 Prestige m of oil the spill; beach other longitude. attachment The stalked barnacle which capitulum bears five large plates. surfacesfloats of 53.8%were Velellaof the specimens were Sargassumattached to bladderstar balls, It is the unique barnacle that constructs its own float, a attachmentfoamy mass surfaces described (Darwin as similar 1851; to Minchin polystyrene, 1996; usingRyan tests (15.3%), floating items as feathers, tar balls and plastic particles as (7.6%) and feathers (2.5%). 20.8% of buoy barnacles lacked an obvious attachment object (Figure 2). theand BritishBranch Islands2012). The and species Baltic Seais widespread (Darwin 1851) in temperate but its sizeThe recorded average for thecapitulum species lengthwas 37 ofmm the in Europeancollected presenceand subtropical in European ocean waters,coast is reaching unusual in(O’ the Riodan NE Atlantic 1967; specimens was 17.7 mm (range 13–22 mm). -

39 DOUZELAGE CONFERENCE SIGULDA 24 April – 27 April 2014
AGROS (CY) ALTEA (E) ASIKKALA (FIN) th BAD KÖTZTING (D) 39 DOUZELAGE CONFERENCE BELLAGIO (I) BUNDORAN (IRL) CHOJNA (PL) GRANVILLE (F) HOLSTEBRO (DK) HOUFFALIZE (B) JUDENBURG (A) SIGULDA KÖSZEG (H) MARSASKALA (MT) MEERSSEN (NL) NIEDERANVEN (L) OXELÖSUND (S) th th PREVEZA (GR) 24 April – 27 April 2014 PRIENAI (LT) SESIMBRA (P) SHERBORNE (GB) SIGULDA (LV) SIRET (RO) SKOFIA LOKA (SI) MINUTES SUŠICE (CZ) TRYAVNA (BG) TÜRI (EST) ZVOLEN (SK) DOUZELAGE – EUROPEAN TOWN TWINNING ASSOCIATION PARTICIPANTS AGROS Andreas Latzias Metaxoula Kamana Alexis Koutsoventis Nicolas Christofi ASIKKALA Merja Palokangos-Viitanen Pirjo Ala-Hemmila Salomaa Miika BAD KOTZTING Wolfgang Kershcer Agathe Kerscher Isolde Emberger Elisabeth Anthofer Saskia Muller-Wessling Simona Gogeissl BELLAGIO Donatella Gandola Arianna Sancassani CHOJNA Janusz Cezary Salamończyk Norbert Oleskow Rafał Czubik Andrzej Będzak Anna Rydzewska Paweł Woźnicki BUNDORAN Denise Connolly Shane Smyth John Campbell GRANVILLE Fay Guerry Jean-Claude Guerry HOLSTEBRO Jette Hingebjerg Mette Grith Sorensen Lene Bisgaard Larsen Victoria Louise Tilsted Joachim Peter Tilsted 2 DOUZELAGE – EUROPEAN TOWN TWINNING ASSOCIATION HOUFFALIZE Alphonse Henrard Luc Nollomont Mathilde Close JUDENBURG Christian Fuller Franz Bachmann Andrea Kober Theresa Hofer Corinna Haasmann Marios Agathocleous KOSZEG Peter Rege Kitti Mercz Luca Nagy Aliz Pongracz MARSASKALA Mario Calleja Sandro Gatt Charlot Mifsud MEERSSEN Karel Majoor Annigje Luns-Kruytbosch Ellen Schiffeleers Simone Borm Bert Van Doorn Irene Raedts NIEDERANVEN Jos -
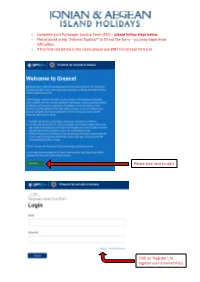
O Complete Your Passenger Locator Form (PLF) – Please Follow Steps Below
o Complete your Passenger Locator Form (PLF) – please follow steps below. o Please avoid using “Internet Explorer” to fill out the form – you may experience difficulties. o If the font size below is too small, please use ctrl+ to increase font size Please click here to start Click on ‘Register’, to register your email address Enter a valid email address Create your password Confirm your password Click “Submit” Please tick all boxes Click “Continue” As you will be flying to Greece, please select Aircraft Click “Continue” Type in the name of the airline e.g. “British Airways” Type in the flight number e.g. “BA0646” Ignore this box Select the date of your arrival Select the destination airport Please note: If you are flying to Preveza (PVK), please select “Aktio” as your point of entry Ignore this box Click “Continue” Type in your last name Type in your first name You can ignore this field Please select Enter your age Select “Passport” Add your number preceded by the country code e.g. “+447123456789” Ignore this box Ignore this box Ignore this box You should be able to see your email address here, if not, please enter it again. Click “Continue” Select country e.g. “United Kingdom” Select region e.g. “London” Type in your city e.g. “London” Type in your postcode Type in your street name Type in your street number Ignore this box Ignore if you have been in the UK for the 14-day period prior to your arrival in Greece. Otherwise, please select the country you visited. -

Parga Hellas
Balancing Economic Development and Environmental Planning for Tourism in Rural Europe DDIIAAGGNNOOSSTTIICC RREEPPOORRTT OOFF PPAARRGGAA September 2001 CONTENTS PAGE INTRODUCTION 1 CHAPTER A. SOCIO-ECONOMIC AND ENVIRONMENTAL PROFILE OF THE AREA 2 A.1. Location A.2. Socio-economic Structure 3 A.2.1 Population – Trends 3 A.2.2 Economic Activities 4 A.2.3. Tourism – In depth Analysis 8 A.2.3.1 Supply 8 A.2.3.2. Tourist Demand 13 A.2.3.3 Problems in the tourism sector 15 A.3. Environmental Protection 16 A.3.1 Designated areas 16 A.3.2. Designated settlements, buildings and monuments 16 A.3.3. Other areas of interest 16 A.3.4. Conclusions 18 A.3.5. Environmental pollution 19 A.4 Access and Accessibility 20 A.5. Stakeholder Analysis 21 A.5.1 Public Authorities 21 A.5.2 NGOs 22 CHAPTER B. THE PLANNING STATUS OF THE AREA 23 B.1 The Structure of settlements 23 B.2 Position of settlements in the regional urban network 23 B.3 The Town Plan 23 CHAPTER C. SWOT ANALYSIS 25 CHAPTER D CONFLICT ANALYSIS 27 Maps of Land Uses 30 References 31 ii LIST OF MAPS Map 1: The Prefecture of Preveza in Epirus 2 Map 2: The Municipality of Parga in Epirus 2 LIST OF TABLES Table A.2.1. Population per Municipality in the Prefecture of Preveza (1961-2001) 3 Table A.2.2. Population per village in the Municipality of Parga (1961-2001) 4 Table A.2.3. Employment per economic sector for the Municipality of Parga (1991) 4 Table A.2.4. -

Marine Litter As Habitat and Dispersal Vector
Chapter 6 Marine Litter as Habitat and Dispersal Vector Tim Kiessling, Lars Gutow and Martin Thiel Abstract Floating anthropogenic litter provides habitat for a diverse community of marine organisms. A total of 387 taxa, including pro- and eukaryotic micro- organisms, seaweeds and invertebrates, have been found rafting on floating litter in all major oceanic regions. Among the invertebrates, species of bryozoans, crus- taceans, molluscs and cnidarians are most frequently reported as rafters on marine litter. Micro-organisms are also ubiquitous on marine litter although the compo- sition of the microbial community seems to depend on specific substratum char- acteristics such as the polymer type of floating plastic items. Sessile suspension feeders are particularly well-adapted to the limited autochthonous food resources on artificial floating substrata and an extended planktonic larval development seems to facilitate colonization of floating litter at sea. Properties of floating litter, such as size and surface rugosity, are crucial for colonization by marine organ- isms and the subsequent succession of the rafting community. The rafters them- selves affect substratum characteristics such as floating stability, buoyancy, and degradation. Under the influence of currents and winds marine litter can transport associated organisms over extensive distances. Because of the great persistence (especially of plastics) and the vast quantities of litter in the world’s oceans, raft- ing dispersal has become more prevalent in the marine environment, potentially facilitating the spread of invasive species. T. Kiessling · M. Thiel Facultad Ciencias del Mar, Universidad Católica del Norte, Larrondo 1281, Coquimbo, Chile L. Gutow Biosciences | Functional Ecology, Alfred-Wegener-Institut Helmholtz-Zentrum für Polar- und Meeresforschung, Bremerhaven, Germany M. -

Download PDF File
Scientific Note Record of coastal colonization of the Lepadid goose barnacle Lepas anatifera Linnaeus, 1758 (Crustacea: Cirripedia) at Arraial do Cabo, RJ 1 1,2 LUIS FELIPE SKINNER & DANIELLE FERNANDES BARBOZA 1Universidade do Estado do Rio de Janeiro (UERJ), Laboratório de Ecologia e Dinâmica Bêntica Marinha, São Gonçalo, Rio de Janeiro. Rua Francisco Portela 1470, Patronato, São Gonçalo, RJ. 24435-005. 2Bolsista PROATEC, UERJ. E-mail: [email protected] Abstract. In this note we record the occurence of Lepas anatifera (Cirripedia) on the hull of a coastal supply boat that operates only in restricted inshore waters of Arraial do Cabo. This species is usually found on tropical and subtropical oceanic waters and, at region, could be classified as transient species. Key words: marine fouling, barnacle, recruitment, inshore transport, species distribution, Cabo Frio upwelling Resumo. Registro de colonização costeira da craca pedunculada Lepas anatifera Linnaeus, 1758 (Crustacea: Cirripedia) na costa de Arraial do Cabo, RJ. Nesta nota nós registramos a ocorrência de Lepas anatifera (Cirripedia) no casco de uma pequena embarcação que opera exclusivamente em águas costeiras de Arraial do Cabo. Esta espécie é típica de águas oceânicas de regiões tropicais e subtropicais e pode ser classificada como transiente na região. Palavras chave: incrustação marinha, craca, recrutamento, transporte costeiro, distribuição de espécies, ressurgência de Cabo Frio Lepas anatifera Linnaeus, 1758 (Fig. 1) is a and not by larval preference. pedunculate goose barnacle with cosmopolitan At Cabo Frio region is frequent to find many distribution (Young 1990, 1998). They are found individuals that arrived to the coast on floating mainly on oceanic waters from tropical and debris. -

Iipebeza B' Preveza B
.-'~-.'.-.-."'--.-.--~----.---- ._ ..•..... _... IIPEBEZA B' PREVEZA B I1paK'LlKU '"COU .6.SUn;pou .6.ls9vou<; LUIl1tocriou 'Yta '"CTJv Icr'"Copia Kat '"COV I10A,l'LlcrIlO '"CTJ<; I1pe~s1,;a<; (16-20 Lcn'tqt~plou 2009) Proceedings of the Second International Symposium for the History and Culture of Preveza (16-20 September 2009) ANATYIIO OFFPRINT E 71:10"'1] flO VIKIj E7I:Iflt},cia Scientific Editors Maplva BpeA.Al]-ZuxOU Marina Vrelli-Zachou Xpi]crwr; L'taupO.KOr; Christos Stavrakos E7I:IfleAcia EK60(J1]r; Editors N1Kor; 11. KapullncAar; Nikos D. Karabelas Michael Stork Michael Stork llANEIIILTHMIO InANNINnN UNIVERSITY OF IOANNINA ~HMOL IIPEBEZAl: MUNICIPALITY OF PREVEZA I~PYMA AKTIA NIKOTIOAIL ACTIA NICOPOLIS FOUNDATION IIPEBEZA 2010 PREVEZA 2010 Copyright © 2010 IIaVE1!IGtl'II.110 Iroavvivrov, "'1'1110<; IIp£pE~a<;, 1op1JJ.la AKTIa N1K01tOA.l<; University ofIoannina, M1lllicipaIity ofPreveza, Actia Nicopolis Foundation IIaVE1!IGTIJJ.l1O 1roavvivrov, IIaVE1!ICITl]J.l101)1tOA.l], 451 10 1roa.vvlva Tl]A..: 26510-06544/ email: [email protected], chstavra@uoLgr MIJ.lO<; IIp£pE~a<;, EA. BEV1~£A.01J & IIaxouJ.ll] 1,481 00 IIp£pE~a Tl]A..: 26820-29889 / email: [email protected] 1opllJ.la AK'tia N1K01tOA.I<;, E9vu(1j<; AVTIG'tUGEro<; 114,481 00 IIp£pE~a Tl]A..: 26820-22233 / email: [email protected] University ofIoanniua, University Campus, 451 10 1oaunina, Greece Tel.: +30-26510-06544 / email: [email protected]@uoi.gr Municipality ofPreveza, El. Venizelou & 1, Pachoumi str., 481 00 Preveza, Greece Tel.: +30-26820-29889 / email: [email protected] Actia Nicopolis FOlmdation, 114, Antistaseos str., 481 00 Preveza, Greece Tel.: +30-26820-22233 / email: [email protected] cISBN 978-960-99475-1-0 [I] 978-960-99475-2-7 [II] 978-960-99475-0-3 [set] Simon MERCIECA The Battle of Preveza 1538: the Knights of Malta's Perspective !ACOMO BOSIO IS KNOWN PRIMARILY for his three-volume history of the Knights of St John with each volume runniug into over 700 pages and each page nearly the size G of an A3 sheet. -

Maps Provisioning| 4.1.1
Project Title: Towards a Common Quality Control and food chain traceability system for the Greek – Italian primary sector of activity Deliverable Title: Maps Provisioning| 4.1.1. Part A. Field data survey and first level analysis Author : TEI of Epirus (LP) Type : Document/ Software /Content Document Reference : Internal / Draft / Final Version : 02 Date : December 15, 2013 AGRO Quality D.4.4.1 Maps Provisioning Control Page Deliverable Number D.4.1.1 Corresponding WP 4 Title Special Purpose GIS development Corresponding Action 4.1. Title Users requirements gathering and functionality Responsible Partner: TEI of Epirus (LP) Working Group Part A. Field data survey and first level analysis Kaltsis Ioannis Papantoniou Trifonas Zampounis Vassilios Lambraki Eleni Myriounis Christos Scientific Coordinator: Georgios Manos, Tsirogiannis Ioannis Creation Date: 01/09/2013 Last Update: 01/09/2013 Type: Document Version: 1 Modification Control VERSION DATE COMMENTARY/STATUS AUTHOR 1 1/9/2013 First draft TEI of Epirus (LP), Kaltsis Ioannis, Papantoniou Trifonas, Zampounis Vassilios, Final (Part A. Field data survey 2 15/12/2013 Lambraki Eleni, Myriounis and first level analysis) Christos Page 2 AGRO Quality D.4.4.1 Maps Provisioning Table of Contents 1 Introduction ..........................................................................................................................................5 2 AGROQuality Maps ...............................................................................................................................5 -

Arta Preveza Lease Area Environmental Report 2019
ARTA PREVEZA LEASE AREA ENVIRONMENTAL REPORT 2019 Contents 1. Introduction ............................................................................................................... 3 2. HSE Studies and Reports ............................................................................................ 5 2.1. Environmental Baseline Reports & Environmental Action Plan ................................ 5 2.2. Environmental Monitoring Program .......................................................................... 8 2.3. Project HSE Plan and Bridging Documentation for the 2D Land Geophysical Survey 9 3. Stakeholders Engagement and Social Monitoring (incl. in the Environmental Action Plan) 11 2 ARTA - PREVEZA LEASE AREA – ENVIRONMENTAL REPORT 2019 HSE Policies & System, Environmental Studies and Implementation 1. Introduction The following are representing the implementation of HELPE’s policies and methodologies related to the Arta-Preveza Lease Area. Hellenic Petroleum has operated during the past in this area as an operator and has gained a deep understanding and knowledge of the context, regulations, local communities’ relations and management that could guarantee a smoother development process in the Lease Area Arta-Preveza with no impact to the environment and the local communities. On March 16th 2018, the Greek State awarded a Lease Agreement for Hydrocarbon Exploration and Production in the onshore area of Arta-Preveza in Western Greece. Hellenic Petroleum, acting as the sole Operator, is fulfilling its commitments and has planned -

Macquarie University PURE Research Management System
Macquarie University PURE Research Management System This is the Accepted Manuscript version of the following article: Shine, R., Goiran, C., Shilton, C., Meiri, S., Brown, G. P. (2020) The life aquatic: an association between habitat type and skin thickness in snakes. Biological Journal of the Linnean Society, vol. 128, no. 4, pp. 975–986. which has been published in final form at: Access to the published version: https://doi.org/10.1093/biolinnean/blz136 Version archived for private and non-commercial use with the permission of the author/s and according to publisher conditions. For further rights please contact the publisher. 1 The Life Aquatic: an association between habitat type and 2 skin thickness in snakes 3 4 RICHARD SHINE1*, CLAIRE GOIRAN2, CATHERINE SHILTON3, 5 SHAI MEIRI4,5 and GREGORY P. BROWN1 6 7 1Department of Biological Sciences, Macquarie University, NSW 2019, Australia 8 2LabEx Corail & ISEA, Université de la Nouvelle-Calédonie, BP R4, 98851 Nouméa cedex, 9 New Caledonia 10 3Berrimah Veterinary Laboratories, Northern Territory Department of Primary Industry and 11 Resources, Darwin, Northern Territory, Australia 12 4School of Zoology, Tel-Aviv University, 6997801 Tel Aviv, Israel 13 5The Steinhardt Museum of Natural History, Tel-Aviv University, 6997801 Tel Aviv, Israel 14 15 *Corresponding author. E-mail: [email protected] 16 17 SHORT RUNNING TITLE: SKIN THICKNESS IN SNAKES 18 19 20 21 Manuscript for consideration in Biological Journal of the Linnean Society 22 3 July 2019 23 1 24 25 An aquatic animal faces challenges not encountered by its terrestrial counterparts, promoting 26 adaptive responses in multiple traits. -

`Degraded' RNA Profiles in Arthropoda and Beyond
‘Degraded’ RNA profiles in Arthropoda and beyond Sean D. McCarthy, Michel M. Dugon and Anne Marie Power School of Natural Sciences, Ryan Institute for Environmental, Marine and Energy Research, National University of Ireland Galway, Ireland ABSTRACT The requirement for high quality/non-degraded RNA is essential for an array of molecular biology analyses. When analysing the integrity of rRNA from the barnacle Lepas anatifera (Phylum Arthropoda, Subphylum Crustacea), atypical or sub- optimal rRNA profiles that were apparently degraded were observed on a bioanalyser electropherogram. It was subsequently discovered that the rRNA was not degraded, but arose due to a ‘gap deletion’ (also referred to as ‘hidden break’) in the 28S rRNA. An apparent excision at this site caused the 28S rRNA to fragment under heat- denaturing conditions and migrate along with the 18S rRNA, superficially presenting a ‘degraded’ appearance. Examination of the literature showed similar observations in a small number of older studies in insects; however, reading across multiple disciplines suggests that this is a wider issue that occurs across the Animalia and beyond. The current study shows that the 28S rRNA anomaly goes far beyond insects within the Arthropoda and is widespread within this phylum. We confirm that the anomaly is associated with thermal conversion because gap-deletion patterns were observed in heat-denatured samples but not in gels with formaldehyde-denaturing. Subjects Aquaculture, Fisheries and Fish Science, Genomics, Marine Biology, Taxonomy, Zoology Keywords Hidden break, Denaturing, Taxonomy, Gap deletion, Degraded rNA, Bioanalyser Submitted 4 July 2015 Accepted 4 November 2015 INTRODUCTION Published 1 December 2015 Anomalies in the gel migration of the 28S subunit rRNA in denatured samples have Corresponding author mostly appeared in the older literature (Applebaum, Ebstein& Wyatt, 1966 ; Ishikawa& Anne Marie Power, [email protected] Newburgh, 1972; Fujiwara& Ishikawa, 1986 ).