IR Investigates: Edible Oils Vibrational Spectroscopy Offers an Alternative for Detecting Adulteration of These Dietary Supplements
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Hazelnut: a Valuable Resource
International Journal of Food Engineering Vol. 6, No. 2, December 2020 Hazelnut: A Valuable Resource Raquel P. F. Guiné and Paula M. Reis Correia CI&DETS/ESAV, Polytechnic Institute of Viseu, Department of Food Industry, Viseu, Portugal Email: [email protected], [email protected] Abstract—Hazel (Corylus avellana L.) nutshell is one of the remaining 90% is used for industrial purposes as shelled most consumed and most appreciated nut fruit all over the nuts [7], [8]. world. It is believed to have constituted a basic food in early Similarly, to other nuts, hazelnuts are included in the prehistory, in temperate zones of the globe, such as for dietary guidelines of several countries, due to their high example Europe. Presently the hazelnut production is nutritional content and bioavailability of those nutrients. mainly concentrated on the Black Sea coast of Turkey, but other countries are also important producers, like for Furthermore, the bioactive molecules present in hazelnuts example Portugal, situated on the western Europe, in the have been reported as having several benefits for the Iberian Peninsula. The objective of this work is to make a human health [9]–[11]. review about the worldwide importance of hazelnut, their usages, including gastronomic and industrial applications, II. CONSUMPTION & MAIN USAGES as well as some ways that allow adding value to this fruit, making it an even more valuable resource. The advantages From the Neolithic era, in the regions of Europe and include higher income for produces, lower environmental the Caucasus, hazelnut has been used for human impacts and valorisation of residues improving consumption. -

Essential Wholesale & Labs Carrier Oils Chart
Essential Wholesale & Labs Carrier Oils Chart This chart is based off of the virgin, unrefined versions of each carrier where applicable, depending on our website catalog. The information provided may vary depending on the carrier's source and processing and is meant for educational purposes only. Viscosity Absorbtion Comparible Subsitutions Carrier Oil/Butter Color (at room Odor Details/Attributes Rate (Based on Viscosity & Absorbotion Rate) temperature) Description: Stable vegetable butter with a neutral odor. High content of monounsaturated oleic acid and relatively high content of natural antioxidants. Offers good oxidative stability, excellent Almond Butter White to pale yellow Soft Solid Fat Neutral Odor Average cold weather stability, contains occlusive properties, and can act as a moistening agent. Aloe Butter, Illipe Butter Fatty Acid Compositon: Palmitic, Stearic, Oleic, and Linoleic Description: Made from Aloe Vera and Coconut Oil. Can be used as an emollient and contains antioxidant properties. It's high fluidiy gives it good spreadability, and it can quickly hydrate while Aloe Butter White Soft Semi-Solid Fat Neutral Odor Average being both cooling and soothing. Fatty Acid Almond Butter, Illipe Butter Compostion: Linoleic, Oleic, Palmitic, Stearic Description: Made from by combinging Aloe Vera Powder with quality soybean oil to create a Apricot Kernel Oil, Broccoli Seed Oil, Camellia Seed Oil, Evening Aloe Vera Oil Clear, off-white to yellow Free Flowing Liquid Oil Mild musky odor Fast soothing and nourishing carrier oil. Fatty Acid Primrose Oil, Grapeseed Oil, Meadowfoam Seed Oil, Safflower Compostion: Linoleic, Oleic, Palmitic, Stearic Oil, Strawberry Seed Oil Description: This oil is similar in weight to human sebum, making it extremely nouirshing to the skin. -
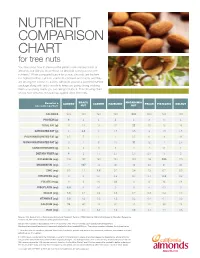
Nutrient Comparison Chart
NUTRIENT COMPARISON CHART for tree nuts You may know how to measure the perfect one-ounce portion of almonds, but did you know those 23 almonds come packed with nutrients? When compared ounce for ounce, almonds are the tree nut highest in fiber, calcium, vitamin E, riboflavin and niacin, and they are among the lowest in calories. Almonds provide a powerful nutrient package along with tasty crunch to keep you going strong, making them a satisfying snack you can feel good about. The following chart shows how almonds measure up against other tree nuts. BRAZIL MACADAMIA Based on a ALMOND CASHEW HAZELNUT PECAN PISTACHIO WALNUT one-ounce portion1 NUT NUT CALORIES 1602 190 160 180 200 200 160 190 PROTEIN (g) 6 4 4 4 2 3 6 4 TOTAL FAT (g) 14 19 13 17 22 20 13 19 SATURATED FAT (g) 1 4.5 3 1.5 3.5 2 1.5 1.5 POLYUNSATURATED FAT (g) 3.5 7 2 2 0.5 6 4 13 MONOUNSATURATED FAT (g) 9 7 8 13 17 12 7 2.5 CARBOHYDRATES (g) 6 3 9 5 4 4 8 4 DIETARY FIBER (g) 4 2 1.5 2.5 2.5 2.5 3 2 POTASSIUM (mg) 208 187 160 193 103 116 285 125 MAGNESIUM (mg) 77 107 74 46 33 34 31 45 ZINC (mg) 0.9 1.2 1.6 0.7 0.4 1.3 0.7 0.9 VITAMIN B6 (mg) 0 0 0.1 0.2 0.1 0.1 0.3 0.2 FOLATE (mcg) 12 6 20 32 3 6 14 28 RIBOFLAVIN (mg) 0.3 0 0.1 0 0 0 0.1 0 NIACIN (mg) 1.0 0.1 0.4 0.5 0.7 0.3 0.4 0.3 VITAMIN E (mg) 7.3 1.6 0.3 4.3 0.2 0.4 0.7 0.2 CALCIUM (mg) 76 45 13 32 20 20 30 28 IRON (mg) 1.1 0.7 1.7 1.3 0.8 0.7 1.1 0.8 Source: U.S. -

Information About Oils the National Edible Oil Distributors’ Association
Information About Oils The National Edible Oil Distributors’ Association Sunflower Oil Sunflower Oil - Information About Oils Introduction Nutritional Profile Whilst the vibrant strong sunflower is recognised Due to its nutritional profile, sunflower oil can bear worldwide for its beauty, it is also an important the following nutrition claims: source of food. Sunflower oil is a valued and Nutrition Claims High polyunsaturated fat (more healthy vegetable oil and sunflower seeds are en- than 45% of the fatty acids are from polyunsaturat- joyed as a healthy, tasty snack and nutritious ingre- ed fats, which represent more than 20% of the en- dient to many foods. ergy content) High unsaturated fat (more than 70% Sunflower oil is obtained from the seeds within the of the fatty acids are from unsaturated fats, which brown hub in the centre of the sunflower plant. represent more than 20% of the energy content) Each flower can develop up to 2000 sunflower High vitamin E (more than 30% of Recommended seeds. The oil is pale yellow in colour but contains Daily Allowances (RDA) of vitamin E set at 12 mg/day a level of natural waxes that give the oil a ‘cloudy’ Health claims – Positive EFSA opinion Linoleic acid appearance at cooler temperatures. These waxes (omega-6 fatty acids) contributes to the mainte- can be removed by a process commonly known as nance of normal blood cholesterol concentrations. winterisation. Essential fatty acids (omega-3 and omega-6 fatty The wild sunflower is native to North America but acids) are needed for the normal growth of children. commercialisation of the plant took place in Rus- Vitamin E protects lipids, proteins and DNA against sia. -

List of Legumes
Healthy Oils & SmokePoints When it comes to the cooking oil in your cupboard, there is a huge difference in healthfulness depending on how the oil is stored and how it will be used. First let’s get some definitions for commonly used terms on labels and discussions about oils. Term Definition Cold Pressed Extracted without using heat. Extracted using a screw-type machine that presses the oil out. Can be done Expeller Pressed slowly, with very little heat, or can be done quickly with lots of friction and high temperatures. The first cold pressing which contains the best tasting and most healthful oils. Must contain less than 1% acids. By definition, this is cold-pressed and first Extra Virgin pressed, so don’t need to see these terms on the label. Must say 100% extra virgin, or may be a blend. The first cold pressing, but can contain a little more acids than the extra-virgin Virgin (1-3 percent). Seeds that have been genetically manipulated to decrease the amount of High Oleic essential fatty acids so that they have a longer shelf life. Are left in their state after pressing – no filtering. These oils tend to be more Unrefined Oils flavorful and richer in nutrients, however they have a very low smoke point. Oils have their impurities filtered out, to increase stability and allow for higher Refined temperature cooking. The processing can use toxic solvents, caustic soda, bleaches and phosphoric acid. Smoke Point Stage at which a heated oil begins to smoke, just before it bursts into flames. HEALTHY OILS & SMOKE POINTS PAGE | 1 © 2021 Health-Naturally.org Term Definition Oil should smell and taste like the food it came from. -

Medicinal Use of Sunflower Oil and Present Status of Sunflower in Pakistan: a Review Study
Sci., Tech. and Dev., 31 (2): 99-106, 2012 Medicinal Use of Sunflower Oil and Present Status of Sunflower in Pakistan: A Review Study MUHAMMAD ARSHAD AND MUHAMMAD AMJAD Oilseeds Research Program, National Agricultural Research Centre, Islamabad, Pakistan. Abstract Sunflower {Helianthus annus (L.)} contributes 30% in domestic edible oil crop and has become the most important oil crop. A case study was planned to examine and review the current status of its production in Pakistan and sunflower oil use in medicine and nutrition. This study deals with the medicinal values of its oil with regards to its benefits and side effects. Sunflower seeds contain 20-30% protein as well as iron, B vitamins, vitamin A, calcium, nitrogen and phosphorus. The constituents of the seeds are a volatile oil, carbonate of potash, tannin and excellent sources of the B vitamins (B1, B3 and B6) including niacin and pantothenate. Sunflower oil is high in the essential vitamin E and low in saturated fat. Two common types of sunflower oil are linoleic and high oleic. Linoleic oil has high levels of polyunsaturated fat. It is also known for having a clean taste and low levels of trans fat. High oleic sunflower oils are classified as having monounsaturated levels of 80% and above. The iron-rich sunflower seeds are, by weight, 47% fat and 20-30 % protein. The seeds have more than 48 calories/tablespoon. Sunflower seeds have more iron than any other food except for liver and egg yolk. Sunflower seeds are more commonly eaten as a healthy snack than as part of a meal. -

Effect of Sunlight on Quality and Stability of Dietary Oils and Fats
Pak. J. Biochem. Mol. Biol. 2010; 43(3):123-125 Effect of sunlight on quality and stability of dietary oils and fats Muhammad Sohail1*, Taufiq Ahmed2, Saeed Akhtar3 and Yasser Durrani3 1Department of Food Science and Technology, NWFP Agricultural University, Peshawar, Pakistan 2Nuclear Institute for Food and Agricultural (NIFA), Tarnab, Peshawar, Pakistan 3Food Biochemistry Section, PCSIR Labs Complex, Peshawar, Pakistan Abstract: The present study was conducted to evaluate nine oil and fat samples i.e. animal fat (A.F), vanaspati ghee (V.G), sunflower oil (SFO), canola oil (Can.O), desi ghee (D.G), rapeseed oil (RPS), soybean oil (SBO), sea buckthorn seed oil (S.B.Seed) and sea buckthorn pulp oil (S.B.Pulp) for their quality and stability under sunlight condition. The quality of samples was determined in terms of peroxide value (POV), free fatty acid (FFA), beta-carotene content and colour (O.D) at the start of five weeks experiment followed by weekly analysis. The maximum POV increase was found for V.G (7040.48 %) and minimum increase was found for S.B.Seed oil (195.43 %). The maximum FFA increase was found in SFO (545.45 %) while the minimum increase was found in R.P.S (34.33%). The maximum decrease of beta-carotene was found in SFO (98.78 %) while the minimum decrease was found in desi ghee (62.91%). The maximum reduction in O.D was found for canola oil (84.91 %) whereas the minimum decrease was found in SFO (62.5%). Results indicated that light affected the dietary oils and fats very much therefore great care should be taken during their storage in order to avoid them from rancidity. -

Hazelnut Butter and Coffee Meringues
Hazelnut Butter and Coffee Meringues When you need a “WOW” total show stopper dessert, this is it. Light and airy Hazlenut Butter and coffee Meringues are as wonderful to eat as they are to look at. For a high-contrast swirl, go easy when folding in the nut butter. A stroke or two with the spatula is enough. Ingredients MAKES ABOUT 24 SERVINGS 1 cup Farm Fresh Nuts Raw Filberts/Hazelnuts ¼ teaspoon kosher salt 4 large egg whites Pinch of cream of tartar ½ cup granulated sugar ¾ cup powdered sugar 2 tablespoons coffee beans, chopped Preparation DO AHEAD: Step 1 Preheat oven to 350°. Toast hazelnuts on a rimmed baking sheet, tossing once, until golden brown, 10–12 minutes. Remove nuts and reduce oven temperature to 200°. Bundle nuts in a kitchen towel and rub vigorously to remove skins. Spread out and let cool. Step 2 Blend hazelnuts and salt in a food processor until a smooth, creamy nut butter forms (it should be pretty fluid; keep processing if still stiff); set aside. Step 3 Using an electric mixer on high speed, beat egg whites and cream of tartar until frothy, about 1 minute. With motor running, gradually add granulated sugar and beat until medium peaks form, about 5 minutes. Gradually add powdered sugar and continue to beat until stiff, glossy peaks form, 8–10 minutes. Step 4 Transfer meringue to a large bowl and gently fold in half of reserved hazelnut butter, leaving plenty of streaks. Add remaining hazelnut butter and fold once just to barely blend. Mixture should be marbled with thick ribbons of nut butter. -

Hazelnut: a Valuable Resource
International Journal of Food Engineering Vol. 6, No. 2, December 2020 Hazelnut: A Valuable Resource Raquel P. F. Guiné and Paula M. Reis Correia CI&DETS/ESAV, Polytechnic Institute of Viseu, Department of Food Industry, Viseu, Portugal Email: [email protected], [email protected] Abstract—Hazel (Corylus avellana L.) nutshell is one of the remaining 90% is used for industrial purposes as shelled most consumed and most appreciated nut fruit all over the nuts [7], [8]. world. It is believed to have constituted a basic food in early Similarly, to other nuts, hazelnuts are included in the prehistory, in temperate zones of the globe, such as for dietary guidelines of several countries, due to their high example Europe. Presently the hazelnut production is nutritional content and bioavailability of those nutrients. mainly concentrated on the Black Sea coast of Turkey, but other countries are also important producers, like for Furthermore, the bioactive molecules present in hazelnuts example Portugal, situated on the western Europe, in the have been reported as having several benefits for the Iberian Peninsula. The objective of this work is to make a human health [9]–[11]. review about the worldwide importance of hazelnut, their usages, including gastronomic and industrial applications, II. CONSUMPTION & MAIN USAGES as well as some ways that allow adding value to this fruit, making it an even more valuable resource. The advantages From the Neolithic era, in the regions of Europe and include higher income for produces, lower environmental the Caucasus, hazelnut has been used for human impacts and valorisation of residues improving consumption. -

Vegetable Oils
Vegetable oils Vegetable oils are obtained from plants. They are important ingredients in many foods, and can be hardened through a chemical process to make, for example, margarine. They can also be used as fuels, for example as biodiesel. Emulsifiers are food additives that prevent oil and water mixtures in food from separating. Vegetable oils are natural oils found in seeds, nuts and some fruit. These oils can be extracted. The plant material is crushed and pressed to squeeze the oil out. Olive oil is obtained this way. Sometimes the oil is more difficult to extract and has to be dissolved in a solvent. Once the oil is dissolved, the solvent is removed by distillation, and impurities such as water are also removed, to leave pure vegetable oil. Sunflower oil is obtained in this way. Structure of vegetable oils Molecules of vegetable oils consist of glycerol and fatty acids. In the diagram below you can see how three long chains of carbon atoms are attached to a glycerol molecule to make one molecule of vegetable oil. The structure of a vegetable oil molecule You do not need to know any details about the structure of vegetable oil molecules for the exam. Vegetable oils have higher boiling points than water. This means that foods can be cooked or fried at higher temperatures than they can be cooked or boiled in water. Food cooked in vegetable oils: cook faster than if they were boiled have different flavours than if they were boiled. However, vegetable oils are a source of energy in the diet. -

Food Oils – List
Ver 01 – Nov 21, 2016 Lope Llamazares FOOD OILS – LIST: Packaging: Kosher Oil: Code: Description: HS Code : RBDW grade. Practically odorless oil. 23kg/200kg/930kg Source of essential fatty acids, Monounsaturated and polyunsaturated 8557 21MT Flexitanks Yes Sunflower oil fats. Low saturated fat levels. HS Code: 15121990 It also has beneficial amounts of lecithin. RBDW grade. Practically odorless oil Sunflower oil – 23kg/200kg/930kg Source of essential fatty acids, Monounsaturated and polyunsaturated Linoleic 8099 21MT Flexitanks Yes fats. Low saturated fat levels. HS Code: 15121990 It also has beneficial amounts of lecithin. Linoleic content: aprox 60% RBDW grade. Practically odorless oil. Source of essential fatty acids, Monounsaturated and polyunsaturated 23kg/200kg/930kg Sunflower oil – High fats. Low saturated fat levels. 8553 21MT Flexitanks Yes It also has beneficial amounts of lecithin. Oleic oil HS Code: 15121990 Greater stability and resistance to rancidity Oleic content: aprox 80% 23kg/200kg/930kg Vitamin E source. High content of Linoleic & Oleic acids. Grapeseed oil 0827 21MT Flexitanks Yes Frutty like taste ideal for marinate meat and prepare vinaigrettes . HS Code: 15159099 Considered suitable for Vegan diets, Lactose Free, Gluten Free, 23kg/200kg/930kg Glutamate Free, BSE Free. 8555 21MT Flexitanks Yes Olive oil pure (99/1) Olive oil composed of refined olive oils and virgin olive oils HS Code: 15099000 Part of any healthy diet. EVOO is substantially rich in monounsaturated fats . Part of any healthy diet. EVOO is used as a food condiment, in salad 23kg/200kg/930kg dressings, and for sautéing. Ext.Virgin Olive oil 8554 21MT Flexitanks Yes EVOO is considered suitable for Vegan diets, Lactose Free, Gluten Free, HS Code: 15091090 Glutamate Free, BSE Free. -

Quantification of Rice Bran Oil in Oil Blends
GRASAS Y ACEITES, 63 (1), ENERO-MARZO, 53-60, 2012, ISSN: 0017-3495 DOI: 10.3989/gya.033311 Quantification of rice bran oil in oil blends By R. Mishra*, H.K. Sharma and G. Sengar Food Engineering and Technology Department. Sant Longowal Institute of Engineering & Technology (Deemed to be University) Longowal – 148 106. Sangrur (PUNJAB). INDIA *Corresponding author: [email protected] RESUMEN ultrasonic velocity and methods based on physico-chemical parameters. The physicochemical parameters such as Cuantificación de aceite de salvado de arroz en mez- ultrasonic velocity, relative association and acoustic clas de aceites. impedance at 2 MHz, iodine value, palmitic acid content and oryzanol content reflected significant changes with Se analizaron diversos parámetros físico-químicos pa- increased proportions of PRBO in the blended oils. These ra la evaluación de mezclas de aceites en diferentes pro- parameters were selected as dependent parameters and % porciones que incluyen: aceite de salvado de arroz físíca- PRBO proportion was selected as independent parameters. mente refinado (PRBO): aceite de girasol (SNF) y las The study revealed that regression equations based on mezclas PRBO: aceite de cártamo (SAF) en diferentes pro- the oryzanol content, palmitic acid composition, ultrasonic porciones. La cuantificación de la presencia del aceite de velocity, relative association, acoustic impedance, and salvado de arroz en las mezclas se llevó a cabo por dife- iodine value can be used for the quantification of rice bran rentes métodos, como cromatografía de gases (GC), cro- oil in blended oils. The rice bran oil can easily be quantified matografía líquida (HPLC), ultrasonidos y métodos basa- in the blended oils based on the oryzanol content by HPLC dos en otros parámetros físico-químicos.