Genesis and Solvus Relations of Submicroscopically Intergrown
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

PARAGONITE PSEUDOMORPHS AFTER KYANITE from TURKEY HEAVEN MOUNTAIN, CLEBURNE COUNTY, ALABAMA1 Tnonnron L. Noarnnnu, Geological Su
THE AMERICAN MINERALOGIST, VOL, 50, MAY_JIJ'}IE, 1965 PARAGONITE PSEUDOMORPHS AFTER KYANITE FROM TURKEY HEAVEN MOUNTAIN, CLEBURNE COUNTY, ALABAMA1 TnonNroN L. Noarnnnu, GeologicalSuraey of Alabama, Uniaersity, Alabama. ABSTRACT Paragonite pseudomorphs after kyanite have been found in the Turkey Heaven Moun- tain kyanite prospects. The pseudomorphs range in size from microscopic grains to mega- scopic prisms, which resemble andalusite crystals Optical, o-ray and petrographic studies indicate direct hysterogenic paragonite alteration of the kyanite. INrnolucrroN An investigationof kyanite prospectsin Sections22 and29,T.17 5., R. 11 E., at Turkey Heaven Mountain, Cleburne County, Alabama, discloseda unique mineral occurrence.The mineral assemblageincludes hysterogenicparagonite pseudomorphsafter kyanite, which resemble andalusite.The geneticphysicochemical parameters are consideredas a function of retrogrademetamorphism within a restrictive water environ- ment. GBor-ocrc SB:rrrNc The Turkey Heaven Mountain areais underlain by two metamorphic rock units: 1) the WedoweeFormation, a highly resistantgraphite-mica schist,and 2) a unit of the Ashland Mica Schist,an extensivelyweathered garnet-muscovite schist. Fresh rock exposuresindicate a retrograde metamorphic cycle from an almandine-amphibolite facies to a green- schist facies. At the crest of Turkey Heaven Mountain, the Wedowee Formation over-liesthe Ashland Mica Schist.The resistantgraphite-mica schist of the WedoweeFormation and associatedquartz veins contribute to the physiographic configuration of the area. The generalregional strike in the Alabama crystalline belt is about N. 45o E., and the dip is generally southeast.The crystalline area has undergoneprimary deformationfrom the stressesapplied generallyfrom the southeast,which producedisoclinal folding and thrust faulting to the northwest. Geologic studies at Turkey Heaven Mountain indicate a secondperiod of deformation in which the stressdirection can be ex- pressedaS a northeast shear couple that formed nonplanar, non-cylin- drical left lateral drag folds. -

List of Abbreviations
List of Abbreviations Ab albite Cbz chabazite Fa fayalite Acm acmite Cc chalcocite Fac ferroactinolite Act actinolite Ccl chrysocolla Fcp ferrocarpholite Adr andradite Ccn cancrinite Fed ferroedenite Agt aegirine-augite Ccp chalcopyrite Flt fluorite Ak akermanite Cel celadonite Fo forsterite Alm almandine Cen clinoenstatite Fpa ferropargasite Aln allanite Cfs clinoferrosilite Fs ferrosilite ( ortho) Als aluminosilicate Chl chlorite Fst fassite Am amphibole Chn chondrodite Fts ferrotscher- An anorthite Chr chromite makite And andalusite Chu clinohumite Gbs gibbsite Anh anhydrite Cld chloritoid Ged gedrite Ank ankerite Cls celestite Gh gehlenite Anl analcite Cp carpholite Gln glaucophane Ann annite Cpx Ca clinopyroxene Glt glauconite Ant anatase Crd cordierite Gn galena Ap apatite ern carnegieite Gp gypsum Apo apophyllite Crn corundum Gr graphite Apy arsenopyrite Crs cristroballite Grs grossular Arf arfvedsonite Cs coesite Grt garnet Arg aragonite Cst cassiterite Gru grunerite Atg antigorite Ctl chrysotile Gt goethite Ath anthophyllite Cum cummingtonite Hbl hornblende Aug augite Cv covellite He hercynite Ax axinite Czo clinozoisite Hd hedenbergite Bhm boehmite Dg diginite Hem hematite Bn bornite Di diopside Hl halite Brc brucite Dia diamond Hs hastingsite Brk brookite Dol dolomite Hu humite Brl beryl Drv dravite Hul heulandite Brt barite Dsp diaspore Hyn haiiyne Bst bustamite Eck eckermannite Ill illite Bt biotite Ed edenite Ilm ilmenite Cal calcite Elb elbaite Jd jadeite Cam Ca clinoamphi- En enstatite ( ortho) Jh johannsenite bole Ep epidote -

What We Know About Subduction Zones from the Metamorphic Rock Record
What we know about subduction zones from the metamorphic rock record Sarah Penniston-Dorland University of Maryland Subduction zones are complex We can learn a lot about processes occurring within active subduction zones by analysis of metamorphic rocks exhumed from ancient subduction zones Accreonary prism • Rocks are exhumed from a wide range of different parts of subduction zones. • Exhumed rocks from fossil subduction zones tell us about materials, conditions and processes within subduction zones • They provide complementary information to observations from active subduction systems Tatsumi, 2005 The subduction interface is more complex than we usually draw Mélange (Bebout, and Penniston-Dorland, 2015) Information from exhumed metamorphic rocks 1. Thermal structure The minerals in exhumed rocks of the subducted slab provide information about the thermal structure of subduction zones. 2. Fluids Metamorphism generates fluids. Fossil subduction zones preserve records of fluid-related processes. 3. Rheology and deformation Rocks from fossil subduction zones record deformation histories and provide information about the nature of the interface and the physical properties of rocks at the interface. 4. Geochemical cycling Metamorphism of the subducting slab plays a key role in the cycling of various elements through subduction zones. Thermal structure Equilibrium Thermodynamics provides the basis for estimating P-T conditions using mineral assemblages and compositions Systems act to minimize Gibbs Free Energy (chemical potential energy) Metamorphic facies and tectonic environment SubduconSubducon zone metamorphism zone metamorphism Regional metamorphism during collision Mid-ocean ridge metamorphism Contact metamorphism around plutons Determining P-T conditions from metamorphic rocks Assumption of chemical equilibrium Classic thermobarometry Based on equilibrium reactions for minerals in rocks, uses the compositions of those minerals and their thermodynamic properties e.g. -

Minerals Found in Michigan Listed by County
Michigan Minerals Listed by Mineral Name Based on MI DEQ GSD Bulletin 6 “Mineralogy of Michigan” Actinolite, Dickinson, Gogebic, Gratiot, and Anthonyite, Houghton County Marquette counties Anthophyllite, Dickinson, and Marquette counties Aegirinaugite, Marquette County Antigorite, Dickinson, and Marquette counties Aegirine, Marquette County Apatite, Baraga, Dickinson, Houghton, Iron, Albite, Dickinson, Gratiot, Houghton, Keweenaw, Kalkaska, Keweenaw, Marquette, and Monroe and Marquette counties counties Algodonite, Baraga, Houghton, Keweenaw, and Aphrosiderite, Gogebic, Iron, and Marquette Ontonagon counties counties Allanite, Gogebic, Iron, and Marquette counties Apophyllite, Houghton, and Keweenaw counties Almandite, Dickinson, Keweenaw, and Marquette Aragonite, Gogebic, Iron, Jackson, Marquette, and counties Monroe counties Alunite, Iron County Arsenopyrite, Marquette, and Menominee counties Analcite, Houghton, Keweenaw, and Ontonagon counties Atacamite, Houghton, Keweenaw, and Ontonagon counties Anatase, Gratiot, Houghton, Keweenaw, Marquette, and Ontonagon counties Augite, Dickinson, Genesee, Gratiot, Houghton, Iron, Keweenaw, Marquette, and Ontonagon counties Andalusite, Iron, and Marquette counties Awarurite, Marquette County Andesine, Keweenaw County Axinite, Gogebic, and Marquette counties Andradite, Dickinson County Azurite, Dickinson, Keweenaw, Marquette, and Anglesite, Marquette County Ontonagon counties Anhydrite, Bay, Berrien, Gratiot, Houghton, Babingtonite, Keweenaw County Isabella, Kalamazoo, Kent, Keweenaw, Macomb, Manistee, -

The Reaction Albite = Jadeite * Quartz Determined Experimentally in The
American Mineralogkt, Volume 65, pages 129-134, 1980 The reaction albite = jadeite * quartz determinedexperimentally in the range600-1200.c TrMorHy J. B. Hou-eNo Departmentof the GeophysicalSciences, University of Chicago Chicago,Illinois 60637 Abstract The reaction NaAlSirOu+ SiO2 :NaAlSirOs jadeite quaftz high albite has been determinedby direct experimentin the temperaturerange 6fi)-1200"C, and the P- T line can be describedby the equation P: 0.35+ 0.0265?("C) + 0.50kbar The brackets,when comparedwith the gas-apparatusdeterminations of this reactionby Hays and Bell (1973)and by Birch and LeComte (1960),show clearly that pressurecorrections are vanishingly small when the NaCl pressurecell is used with the piston-+ylinder device if pis- ton-out methodsare used.The slope(dP/df : 26.5bar oC-t), io conjunction with tabulated entropiesfor quartz andjadeite, leadsto a value for the entropy ofsynthetic high albite. The disorderingentropy of albite is (14.2+2.4J K-' mol-'), and the enthalpy is (12950=3320J mol-t). The disorderingenthalpy is in excellentagreement with recentcalorimetric measure- ments on Amelia albite and its heat-treatedmodification. The best valuesfrom phase equi- libria for the 298 K, I bar, thermochemicaldata for synthetichigh albite are AJI3I".rt*: -3922170+ 4300J mol-' S&r., o- :221.6 + 2.5J K-t mol-' The lack of curvatureof the reaction in the P-T plane imFlies that high albite doesnot un- dergo ordering down to 600'C, at least within the duration time of the experiments.This is also conflrmed by the X-ray patternsof albites from runs at 600"C. Introduction which high_temperature calorimetry (Holm and The stability of the end-member feldspars is of Kleppa, 1968) predicts a slope of 27.6 bar deg-', as fundamental importance to petrology, yet consid- shown later. -

Supplementary Table 1
Supplementary Table 1. Compositional groups, typical sample numbers and location with their bulk compositional, mineralogical and petrographic characteristics at different metamorphic grades. Sample #s, Mineral assemblage, Compositio N/E Traverse, Texture Metamorphic Bulk Chemical mode nal GPS and grade Characteristics and Group coordinates other comments mineral chemistry Ms-Chl-Qtz-Ilm-Mag-Gr ± Kfs ± Pl Mode: (Qtz 35-42%, Ms 30 - 33%, Chl 25 - 30%, Pl 5-7%). #1/00, E, Lithology: Chlorite quartz phyllite (minor white 27 o11.268, mica), white mica quartz phyllite (minor chlorite) /88 o38.581 Two compositional and chlorite bearing quartzite. Millimeter to centimeter groups (one richer in scale banding into M- Al 2O3 ~25 wt.%, Muscovite: domain (mica + chlorite Chlorite Normal #187/01, another ~20 Wt.%). rich), grain size 2 - 20 m 187/3, Rocks richer in Paragonite 5%, Pyrophyllite 19%, Celadonite 12%, and Q - domain (quartz - 187/3/ CHAKRA Al 2O3 are relatively Fe-celadonite 13%. feldspar rich), grain size 10 , N, GPS not less ferruginous (see - 50 µm. known Fig 3). Chlorite: XMg (Mg/(Mg+Fe(tot)) = 0.37 - 0.41. Plagioclase: XAn = 0.03 #ID/00,E, Ms-Chl-Qtz-Bt - Pl ± Ilm ± Tur ± Gr 27 o11.268, /88 o38.581 Chl - Qtz - Ms - Ilm -Gr - Tur - Bt - Pl #2/00, E, Two subgroups - 27 o11.465, high Al, low Fe (18 o /88 38.650 - 24 wt% Al 2O3, - Mode: (Qtz 35-42%, Ms 30 - 33%, Bt 15 - 18%, Chl 4.5-5 wt% Fe 2O3) 10 - 12%, Pl 5-7%). Pervasive foliation defined #3/00, E, and low Al, high Fe by biotite, white mica, o 27 11.465, (14.5 - 17 wt% chlorite and occasional o /88 38.692 Al 2O3, 6.0 - 8.0 wt% Muscovite: ilmenite (S 2) almost parallel Fe 2O3). -

Predictive Mineral Discovery COOPERATIVE RESEARCH CENTRE Scale-Integrated Alteration Studies at Kanowna Belle Dr
predictive mineral discovery COOPERATIVE RESEARCH CENTRE Scale-Integrated Alteration Studies at Kanowna Belle Dr. Glen Masterman and Scott Halley (Placer Dome Asia Pacific) John Walshe (CSIRO) KB Sulfide Evolution KB Sulfur Isotopes YilgarnSulphurIsotopes_AMG by d34s Kanowna Belle 4 to 20.5 (115) -1 to 4 (415) -4 to -1 (175) 1000 -41.6 to -4 (233) 100 dirty 10 zoned clean Au ppm 1 unclassified 0.1 0.01 -8 -4 0 4 8 δ34S N •Dirty pyrite cores with Au, Te, basemetal & Kanowna Belle; Sulfur Isotope values gangue inclusions 40 KB •As-rich and As-poor growth bands 35 •Clean inclusion-free euhedral pyrite 30 2 km 25 KB overgrowths 20 Regional •Late Au, Te & sulfosalt infill (remobilised or 15 •High Au grades correlate with -ve δ34S values in dirty pyrite cores. new addition??) No. of measurements 10 34 5 •δ S values in zoned pyrite are between -2.8 and 2.9 per mil. 0 •Clean pyrite rims are -2.5 to 0.3 per mil. 0 2 4 6 8 -8 -6 -4 -2 10 -12 -10 δ34S •KB pyrites record a history of oxidised fluids early in the evolution of the deposit gradually becoming swamped by reduced fluids. •Regional δ34S values are clustered around 2 per mil KB Alteration Geochemistry Lowes Shoot Alkali Signature (GDD438) Lowes Shoot Pathfinder Signature (GDD438) KB Regional Alkali Halo Muscovite V-rich + alkali feldspar altered samples Albite KB Muscovite-albite tie line 2 km A plot of K/Al vs. Na/Al molar ratios quantifies alteration Pathfinder elements typically associated with Au include W, Mo, mineralogy. -

Clay Minerals
CLAY MINERALS CD. Barton United States Department of Agriculture Forest Service, Aiken, South Carolina, U.S.A. A.D. Karathanasis University of Kentucky, Lexington, Kentucky, U.S.A. INTRODUCTION of soil minerals is understandable. Notwithstanding, the prevalence of silicon and oxygen in the phyllosilicate structure is logical. The SiC>4 tetrahedron is the foundation Clay minerals refers to a group of hydrous aluminosili- 2 of all silicate structures. It consists of four O ~~ ions at the cates that predominate the clay-sized (<2 |xm) fraction of apices of a regular tetrahedron coordinated to one Si4+ at soils. These minerals are similar in chemical and structural the center (Fig. 1). An interlocking array of these composition to the primary minerals that originate from tetrahedral connected at three corners in the same plane the Earth's crust; however, transformations in the by shared oxygen anions forms a hexagonal network geometric arrangement of atoms and ions within their called the tetrahedral sheet (2). When external ions bond to structures occur due to weathering. Primary minerals form the tetrahedral sheet they are coordinated to one hydroxyl at elevated temperatures and pressures, and are usually and two oxygen anion groups. An aluminum, magnesium, derived from igneous or metamorphic rocks. Inside the or iron ion typically serves as the coordinating cation and Earth these minerals are relatively stable, but transform- is surrounded by six oxygen atoms or hydroxyl groups ations may occur once exposed to the ambient conditions resulting in an eight-sided building block termed an of the Earth's surface. Although some of the most resistant octohedron (Fig. -

CHAPTER 4 - MINERAL CHEMISTRY and METAMORPHIC CONDITIONS A
- 217 - CHAPTER 4 - MINERAL CHEMISTRY AND METAMORPHIC CONDITIONS a - 218 - Mineral chemistry and metamorphic conditions The aims of this chapter are to present mineral chemical data, to describe the mineral chemistry in relation to parageneses in selected lithologies (with particular reference to high-pressure metamorphic types) and to evaluate the metamorphic conditions under which these parageneses formed in the light of published experimentally and theoretically derived phase equilibria. Finally, a synthesis of pressure-temperature-time (P-T-t) relationships will be presented. Analytical methods and mineral recalculation schemes are described in Appendices 1 and 2. Results of geothermometry calculations are sumarised in Tbj 4.20. SECTION 4.01 The Eclogites 4.01.1 Summary of petrography Detailed petrographic descriptions appear in Chapter 2, the salient features of which are summarised here. The most common parageneses in the eclogites are:- garnet + omphacite +1- amphibole +1- rutile garnet + omphacite + phengite +1- quartz +1- rutile garnet + omphacite + phengite + zoisite +/_ quartz +/_ rut i e. S - 219 - garnet + omphacite + phengite + zoisite + kyanite +1- quartz ^1- rutile Segregations of zoisite + kyanite + quartz occur in some more aluminous types. Occasionally, Na-poor rocks have pale green or blue-green amphibole in textural equilibrium with omphacite and garnet (figure 2.18) which may become an essential or dominant phase, to give:- garnet + amphibole + clinopyroxene +/_ rutile. The phases listed above will be referred to as 'matrix' phases. Solid inclusions are common in the matrix phases (figures 2.19, 2.45). They are not associated with fractures in the garnets and commonly occur in samples where no alteration of garnet rims or matrix grains has occurred. -
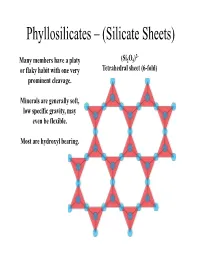
Phyllosilicates – (Silicate Sheets)
Phyllosilicates – (Silicate Sheets) 2- Many members have a platy (Si2O5) or flaky habit with one very Tetrahedral sheet (6-fold) prominent cleavage . Minerals are generally soft, low specific gravity, may even be flexible. Most are hydroxyl bearing. Each tetrahedra is bound to three neiggghboring tetrahedra via three basal bridging oxygens. The apical oxygen of each tetrahedral in a sheet all point in the same direction. The sheets are stacked either apice-to- apice or base-to-base. In an undistorted sheet the hydroxyl (OH) group sits in the centre and each outlined triangle is equivalent. Sheets within sheets…. Apical oxygens, plus the –OH group, coordinate a 6-fold (octahedral) site (XO6). These octahedral sites form infinitely extending sheets. All the octahedra lie on triangular faces, oblique to the tetrahedral sheets. The most common elements found in the 6 -fold site are Mg (or Fe) or Al . Dioctahedral vs Trioctahedral Mg and Al have different charges, but the sheet must remain charge neutral . With 6 coordinating oxygens, we have a partial charge of -6. How many Mg2+ ions are required to retain neutrality? How many Al3+ ions are required to retain neutrality? Mg occupies all octahedral sites, while Al will only occupy 2 out of every 3. The stacking of the sheets dictates the crystallography and c hem istry o f eac h o f t he p hhyll llosili cates. Trioctahedral Dioctahedral O Brucite Gibbsite Hydroxyl Magnesium Aluminium Trioctahedral Is this structure charge neutral? T O T Interlayer Cation T O T Potassium (K+) Phlogopite (Mg end-member biotite) Dioctahedral Is this structure charge neutral? T O T Interlayer Cation T O T Potassium (K+) Muscovite Compositional variation in phyllosilicates There is little solid solution between members of the dioctahedral and trioctahedral groups. -

An EMP and TEM-AEM Study of Margarite, Muscovite
JOURNAL OF PETROLOGY VOLUME 37 NUMBER 2 PAGES 201-233 1996 ANNE FEENSTRA* MINERALOGISCH-PETROGRAPHISCHES INSTITUT, UNIVERSITY OF BERNE, BALTZERSTRASSE 1, CH-J012 BERNE, SWITZERLAND An EMP and TEM-AEM Study of Margarite, Muscovite and Paragonite in Polymetamorphic Metabauxites of Naxos (Cyclades, Greece) and the Implications of Fine-scale Mica Interlayering and Multiple Mica Generations Coexisting white micas and plagioclase were studied by electron covite-paragonite solvus thermometry. Chemical data for Co— microprobe (EMP), and transmission and analytical electron Na micas from this study and literature data indicate that microscopy (TEM-AEM) in greenschist- to amphibolite- naturally coexisting margarite—paragonite pairs display con- grade metabauxites from Naxos. The TEM—AEM studies siderably less mutual solubility than suggested by experimental indicate that sub-micron scale (0-01-1-0 jim thick) semi- work. The variable and irregular Na partitioning between coherent intergrowths ofmargarite, paragonite and muscovite are margarite and muscovite as observed in many metamorphic common up to lower amphibolite conditions. If unrecognized, rocks could largely be related to opposing effects of pressure on such small-scale mica interlayering can easily lead to incorrect Na solubility in margarite and paragonite and/or non-equili- interpretation of EMP data. Muscovite and paragonite in M2 brium between micas. greenschist-grade Naxos rocks are mainly relics of an earlier high-pressure metamorphism (M.\). Owing to the medium- KEY WORDS: Ca—Na—K mica; margarite; mctabauxite; Naxos; sub- pressure M2 event, margarite occurs in middle greenschist-grade micron-scale mica interlayering metabauxites and gradually is replaced by plagioclase + cor- undum in amphibolite-grade metabauxites. The margarite dis- IV VI 3+ VI plays minor Al3 (Fe ,Al)SL3 O-i and considerable INTRODUCTION (Na,K) SiCa-iALj substitution, resulting in up to 44 mol% Since 1970, many new occurrences of the brittle paragonite and 6 mol % muscovite in solution. -

Nomenclature of the Micas
Mineralogical Magazine, April 1999, Vol. 63(2), pp. 267-279 Nomenclature of the micas M. RIEDER (CHAIRMAN) Department of Geochemistry, Mineralogy and Mineral Resources, Charles University, Albertov 6, 12843 Praha 2, Czech Republic G. CAVAZZINI Dipartimento di Mineralogia e Petrologia, Universith di Padova, Corso Garibaldi, 37, 1-35122 Padova, Italy Yu. S. D'YAKONOV VSEGEI, Srednii pr., 74, 199 026 Sankt-Peterburg, Russia W. m. FRANK-KAMENETSKII* G. GOTTARDIt S. GUGGENHEIM Department of Geological Sciences, University of Illinois at Chicago, 845 West Taylor St., Chicago, IL 60607-7059, USA P. V. KOVAL' Institut geokhimii SO AN Rossii, ul. Favorskogo la, Irkutsk - 33, Russia 664 033 G. MOLLER Institut fiir Mineralogie und Mineralische Rohstoffe, Technische Universit/it Clausthal, Postfach 1253, D-38670 Clausthal-Zellerfeld, Germany A. M, R. NEIVA Departamento de Ci6ncias da Terra, Universidade de Coimbra, Apartado 3014, 3049 Coimbra CODEX, Portugal E. W. RADOSLOVICH$ J.-L. ROBERT Centre de Recherche sur la Synth6se et la Chimie des Min6raux, C.N.R.S., 1A, Rue de la F6rollerie, 45071 Od6ans CEDEX 2, France F. P. SASSI Dipartimento di Mineralogia e Petrologia, Universit~t di Padova, Corso Garibaldi, 37, 1-35122 Padova, Italy H. TAKEDA Chiba Institute of Technology, 2-17-1 Tsudanuma, Narashino City, Chiba 275, Japan Z. WEISS Central Analytical Laboratory, Technical University of Mining and Metallurgy, T/'. 17.1istopadu, 708 33 Ostrava- Poruba, Czech Republic AND D. R. WONESw * Russia; died 1994 t Italy; died 1988 * Australia; resigned 1986 wUSA; died 1984 1999 The Mineralogical Society M. RIEDER ETAL. ABSTRACT I I End-members and species defined with permissible ranges of composition are presented for the true micas, the brittle micas, and the interlayer-deficient micas.