Conceptual Model for Managed Wetlands in Suisun Marsh
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
0 5 10 15 20 Miles Μ and Statewide Resources Office
Woodland RD Name RD Number Atlas Tract 2126 5 !"#$ Bacon Island 2028 !"#$80 Bethel Island BIMID Bishop Tract 2042 16 ·|}þ Bixler Tract 2121 Lovdal Boggs Tract 0404 ·|}þ113 District Sacramento River at I Street Bridge Bouldin Island 0756 80 Gaging Station )*+,- Brack Tract 2033 Bradford Island 2059 ·|}þ160 Brannan-Andrus BALMD Lovdal 50 Byron Tract 0800 Sacramento Weir District ¤£ r Cache Haas Area 2098 Y o l o ive Canal Ranch 2086 R Mather Can-Can/Greenhead 2139 Sacramento ican mer Air Force Chadbourne 2034 A Base Coney Island 2117 Port of Dead Horse Island 2111 Sacramento ¤£50 Davis !"#$80 Denverton Slough 2134 West Sacramento Drexler Tract Drexler Dutch Slough 2137 West Egbert Tract 0536 Winters Sacramento Ehrheardt Club 0813 Putah Creek ·|}þ160 ·|}þ16 Empire Tract 2029 ·|}þ84 Fabian Tract 0773 Sacramento Fay Island 2113 ·|}þ128 South Fork Putah Creek Executive Airport Frost Lake 2129 haven s Lake Green d n Glanville 1002 a l r Florin e h Glide District 0765 t S a c r a m e n t o e N Glide EBMUD Grand Island 0003 District Pocket Freeport Grizzly West 2136 Lake Intake Hastings Tract 2060 l Holland Tract 2025 Berryessa e n Holt Station 2116 n Freeport 505 h Honker Bay 2130 %&'( a g strict Elk Grove u Lisbon Di Hotchkiss Tract 0799 h lo S C Jersey Island 0830 Babe l Dixon p s i Kasson District 2085 s h a King Island 2044 S p Libby Mcneil 0369 y r !"#$5 ·|}þ99 B e !"#$80 t Liberty Island 2093 o l a Lisbon District 0307 o Clarksburg Y W l a Little Egbert Tract 2084 S o l a n o n p a r C Little Holland Tract 2120 e in e a e M Little Mandeville -

Desilva Island
SUISUN BAY 139 SUISUN BAY 140 SUISUN BAY SUISUN BAY Located immediately downstream of the confluence of the Sacramento and San Joaquin Rivers, Suisun Bay is the largest contiguous wetland area in the San Francisco Bay region. Suisun Bay is a dynamic, transitional zone between the freshwater input of the Central Valley rivers and the tidal influence of the upper San Francisco Estuary. This area supports a substantial number of nesting herons and egrets, including three of the largest colonies in the region. Although suburban development is rampant along the nearby Interstate 80 corridor to the north, most of the Suisun Bay area is protected from heavy development by the California Department of Fish and Game and a number of private duck clubs. Black- Active Great crowned or year Site Blue Great Snowy Night- Cattle last # Colony Site Heron Egret Egret Heron Egret County active Page 501 Bohannon Solano Active 142 502 Campbell Ranch Solano Active 143 503 Cordelia Road Solano 1998 145 504 Gold Hill Solano Active 146 505 Green Valley Road Solano Active 148 506 Hidden Cove Solano Active 149 507 Joice Island Solano 1994 150 508 Joice Island Annex Solano Active 151 509 Sherman Lake Sacramento Active 152 510 Simmons Island Solano 1994 153 511 Spoonbill Solano Active 154 512 Tree Slough Solano Active 155 513 Volanti Solano Active 156 514 Wheeler Island Solano Active 157 SUISUN BAY 141 142 SUISUN BAY Bohannon Great Blue Herons and Great Egrets nest in a grove of eucalyptus trees on a levee in Cross Slough, about 1.8 km east of Beldons Landing. -

MRA Leadership
2 MRA Leadership President Neil Van Dyke Stowe Mountain Rescue [email protected] July 2010 MRA Member Guides NASA On Undersea Exploration Analog Vice President Mission …………………………………………………………….3 About Steve Chappell……………………………………………...4 Doug Wesson A Letter From Our New President…………………………………4 Juneau Mountain Rescue Suspension Syndrome…………………………..………………….5 [email protected] Commentary from MRA Medical Committee Chair Skeet Glatterer, M.D……………………………………………………...5 Five Colorado SAR Teams Receive Prestigious NASAR Award…6 Past President 2010 MRA Spring Conference Report……………………………..7 Charley Shimanski Book Review: Mountain Responder……………………………….8 [email protected] International Tech Rescue Symposium…………………………….8 Himalayan First: Standby Rescue Helicopters……………………..9 Don‘t Just Do Something—Stand There!.......................................10 National Search and Rescue Week Designated…………………..11 Secretary/Treasurer John Chang Cover photo by NASA. Bay Area Mountain Rescue Unit [email protected] MRA Sponsors At-Large Member Jim Frank Thanks to the corporate supporters of the MRA. Please support Santa Barbara County SAR those that generously support us! Click the logo to follow the [email protected] link! At-Large Member Dave Clarke Portland Mountain Rescue Cell: 503-784-6341 [email protected] Executive Secretary Kayley Trujillo [email protected] Corporate correspondence to: Mountain Rescue Association PO Box 880868 San Diego, CA 92168-0868 ©2010 Mountain Rescue Association All rights reserved. All content ©MRA or as otherwise noted. Permission to reprint granted to MRA units in good standing with the MRA. 3 MRA Member Guides NASA On Undersea Exploration Analog Mission Parts reprinted with permission from NASA From May 10 - 24, 2010, two astronauts, a veteran undersea engineer and an experienced scientist embarked on the 14th NASA Extreme Environment Mission Operations (NEEMO) undersea analog mission at the Aquarius undersea labora- tory. -

Maritime Swamp Forest (Typic Subtype)
MARITIME SWAMP FOREST (TYPIC SUBTYPE) Concept: Maritime Swamp Forests are wetland forests of barrier islands and comparable coastal spits and back-barrier islands, dominated by tall trees of various species. The Typic Subtype includes most examples, which are not dominated by Acer, Nyssa, or Fraxinus, not by Taxodium distichum. Canopy dominants are quite variable among the few examples. Distinguishing Features: Maritime Shrub Swamps are distinguished from other barrier island wetlands by dominance by tree species of (at least potentially) large stature. The Typic Subtype is dominated by combinations of Nyssa, Fraxinus, Liquidambar, Acer, or Quercus nigra, rather than by Taxodium or Salix. Maritime Shrub Swamps are dominated by tall shrubs or small trees, particularly Salix, Persea, or wetland Cornus. Some portions of Maritime Evergreen Forest are marginally wet, but such areas are distinguished by the characteristic canopy dominants of that type, such as Quercus virginiana, Quercus hemisphaerica, or Pinus taeda. The lower strata also are distinctive, with wetland species occurring in Maritime Swamp Forest; however, some species, such as Morella cerifera, may occur in both. Synonyms: Acer rubrum - Nyssa biflora - (Liquidambar styraciflua, Fraxinus sp.) Maritime Swamp Forest (CEGL004082). Ecological Systems: Central Atlantic Coastal Plain Maritime Forest (CES203.261). Sites: Maritime Swamp Forests occur on barrier islands and comparable spits, in well-protected dune swales, edges of dune ridges, and on flats adjacent to freshwater sounds. Soils: Soils are wet sands or mucky sands, most often mapped as Duckston (Typic Psammaquent) or Conaby (Histic Humaquept). Hydrology: Most Maritime Swamp Forests have shallow seasonal standing water and nearly permanently saturated soils. Some may rarely be flooded by salt water during severe storms, but areas that are severely or repeatedly flooded do not recover to swamp forest. -

PESHTIGO RIVER DELTA Property Owner
NORTHEAST - 10 PESHTIGO RIVER DELTA WETLAND TYPES Drew Feldkirchner Floodplain forest, lowland hardwood, swamp, sedge meadow, marsh, shrub carr ECOLOGY & SIGNIFICANCE supports cordgrass, marsh fern, sensitive fern, northern tickseed sunflower, spotted joe-pye weed, orange This Wetland Gem site comprises a very large coastal • jewelweed, turtlehead, marsh cinquefoil, blue skullcap wetland complex along the northwest shore of Green Bay and marsh bellflower. Shrub carr habitat is dominated three miles southeast of the city of Peshtigo. The wetland by slender willow; other shrub species include alder, complex extends upstream along the Peshtigo River for MARINETTE COUNTY red osier dogwood and white meadowsweet. Floodplain two miles from its mouth. This site is significant because forest habitats are dominated by silver maple and green of its size, the diversity of wetland community types ash. Wetlands of the Peshtigo River Delta support several present, and the overall good condition of the vegetation. - rare plant species including few-flowered spikerush, The complexity of the site – including abandoned oxbow variegated horsetail and northern wild raisin. lakes and a series of sloughs and lagoons within the river delta – offers excellent habitat for waterfowl. A number This Wetland Gem provides extensive, diverse and high of rare animals and plants have been documented using quality wetland habitat for many species of waterfowl, these wetlands. The area supports a variety of recreational herons, gulls, terns and shorebirds and is an important uses, such as hunting, fishing, trapping and boating. The staging, nesting and stopover site for many migratory Peshtigo River Delta has been described as the most birds. Rare and interesting bird species documented at diverse and least disturbed wetland complex on the west the site include red-shouldered hawk, black tern, yellow shore of Green Bay. -

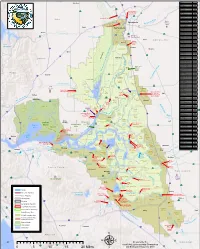
Suisun Marsh Protection Plan Map (PDF)
Proposed County Parks (Hill Slough, Fairfield Beldon’s Landing) Develop passive recreation facilities compatible with Marsh protection (e.g. fishing, picnicking, hiking, nature study.) Boat launching ramp may be constructed Suis nu at Beldon’s Landing. City Suisun Marsh 8 0 etaterstnI 80 a Protection Plan Map flHighway 12 San Francisco Bay Conservation (6) b .J ' and Development Commission I Denverton (7) I December 1976 ) I ~4 Slough Thomasson Shiloh Primary Management Area danyor, Potrero Hills ':__. .---) ... .. ... ~ . _,,. - (8) Secondary Management Area ~ ,. .,,,, Denverton ,,a !\.:r ~ Water-Related Industry Reserve Area c Beldon’s BRADMOOR ISLAND Slough (5) Landing t +{larl!✓' Road Boundary of Wildlife Areas and (9) Ecological Reserves Little I Honker (1) Grizzly Island Unit (9) Bay (2) Crescent Unit (4) Montezuma Slough (3) Island Slough Unit JOICE ISLAND (3) r (4) Joice Island Unit (5) Rush Ranch National Estuarine (10) Ecological Reserve Kirby Hill (6) Hill Slough Wildlife Area Suisun (7) Peytonia Slough Ecological Reserve (8) Grey Goose Unit GRIZZLY ISLAND (2) GRIZZLY ISLAND (9) Gold Hills Unit (10) Garibaldi Unit (11) West Family Unit (12) Goodyear Slough Unit Benicia Area Recommended for Aquisition a. Lawler Property I (11) Hills b. Bryan Property . ~-/--,~ c. Smith Property ,,-:. ...__.. ,, \ 1 Collinsville: Reserve seasonal marshes and Benicia Hills lowland grasslands for their Amended 2011 Grizzly Bay intrinsic value to marsh wildlife and Steep slopes with high landslide and soil to act as the buffer between the erosion potentials. Active fault location. Land (1) Marsh and any future water-related Collinsville Road use practices should be controlled to prevent uses to the east. -

255 Subpart B—First Coast Guard District
SUBCHAPTER G—REGATTAS AND MARINE PARADES PART 100—SAFETY OF LIFE ON 100.703 Special Local Regulations; Recur- ring Marine Events, Sector St. Peters- NAVIGABLE WATERS burg. 100.704 Special Local Regulations; Marine Subpart A—General Events within the Captain of the Port Charleston. Sec. 100.01 Purpose and intent. 100.713 Annual Harborwalk Boat Race; 100.05 Definition of terms used in this part. Sampit River, Georgetown, SC. 100.10 Coast Guard-State agreements. 100.721 Special Local Regulations; Clear- 100.15 Submission of application. water Super Boat National Champion- 100.20 Action on application for event as- ship, Gulf of Mexico; Clearwater Beach, signed to State regulation by Coast FL. Guard-State agreement. 100.724 Annual Augusta Invitational Rowing 100.25 Action on application for event not Regatta; Savannah River, Augusta, GA. assigned to State regulation by Coast 100.732 Annual River Race Augusta; Savan- Guard-State agreement. nah River, Augusta, GA. 100.30 Approval required for holding event. 100.750–100.799 [Reserved] 100.35 Special local regulations. 100.40 Patrol of the regatta or marine pa- Subpart E—Eighth Coast Guard District rade. 100.45 Establishment of aids to navigation. 100.800 [Reserved] 100.50–100.99 [Reserved] 100.801 Annual Marine Events in the Eighth Coast Guard District. Subpart B—First Coast Guard District 100.850–100.899 [Reserved] 100.100 Special Local Regulations; Regattas and Boat Races in the Coast Guard Sec- Subpart F—Ninth Coast Guard District tor Long Island Sound Captain of the 100.900 [Reserved] Port Zone. 100.901 Great Lakes annual marine events. -

Tidal Wetland Gross Primary Production Across the Continental
RESEARCH ARTICLE Tidal Wetland Gross Primary Production Across 10.1029/2019GB006349 the Continental United States, 2000–2019 Special Section: R. A. Feagin1 , I. Forbrich2 , T. P. Huff1, J. G. Barr3, J. Ruiz‐Plancarte4 , J. D. Fuentes4 , Carbon cycling in tidal wet- 4 5 5 6 7 R. G. Najjar , R. Vargas , A. Vázquez‐Lule , L. Windham‐Myers , K. D. Kroeger , lands and estuaries of the con- 8 1 9 8 8 2 tiguous United States E. J. Ward , G. W. Moore , M. Leclerc , K. W. Krauss , C. L. Stagg , M. Alber , S. H. Knox10 , K. V. R. Schäfer11, T. S. Bianchi12 , J. A. Hutchings13 , H. Nahrawi9,14 , 1 1 1 1,15 6 16 Key Points: A. Noormets , B. Mitra , A. Jaimes , A. L. Hinson , B. Bergamaschi , J. S. King , and 17 • We created the Blue Carbon (BC) G. Miao model, which mapped the Gross Primary Production (GPP) of all 1Department of Ecology and Conservation Biology, Texas A&M University, College Station, TX, USA, 2Marine Biological tidal wetlands within the Laboratory, Woods Hole, MA, USA, 3Elder Research, Charlottesville, VA, USA, 4Department of Meteorology and continental United States 5 • Atmospheric Science, Pennsylvania State University, University Park, PA, USA, Department of Plant and Soil Sciences, The BC model provides maps of tidal 6 7 wetland GPP at sub‐250 m scales University of Delaware, Newark, DE, USA, United States Geological Survey, Menlo Park, CA, USA, United States 8 and at 16‐day intervals for the years Geological Survey, Woods Hole, MA, USA, United States Geological Survey, Wetland and Aquatic Research Center, 2000‐2019 Lafayette, LA, USA, 9Atmospheric Biogeosciences, University of Georgia, Athens, GA, USA, 10Department of Geography, • 2 The average daily GPP per m was University of British Columbia, Vancouver, BC, USA, 11Department of Biological Sciences, Rutgers University, Newark, 4.32 ± 2.45 g C/m2/day, and the total NJ, USA, 12Departmet of Geological Sciences, University of Florida, Gainesville, FL, USA, 13Department of Earth and annual GPP for the continental 14 United States was 39.65 ± 0.89 Tg Planetary Sciences, Washington University, St. -

Tidal Marsh Recovery Plan Habitat Creation Or Enhancement Project Within 5 Miles of OAK
U.S. Fish & Wildlife Service Recovery Plan for Tidal Marsh Ecosystems of Northern and Central California California clapper rail Suaeda californica Cirsium hydrophilum Chloropyron molle Salt marsh harvest mouse (Rallus longirostris (California sea-blite) var. hydrophilum ssp. molle (Reithrodontomys obsoletus) (Suisun thistle) (soft bird’s-beak) raviventris) Volume II Appendices Tidal marsh at China Camp State Park. VII. APPENDICES Appendix A Species referred to in this recovery plan……………....…………………….3 Appendix B Recovery Priority Ranking System for Endangered and Threatened Species..........................................................................................................11 Appendix C Species of Concern or Regional Conservation Significance in Tidal Marsh Ecosystems of Northern and Central California….......................................13 Appendix D Agencies, organizations, and websites involved with tidal marsh Recovery.................................................................................................... 189 Appendix E Environmental contaminants in San Francisco Bay...................................193 Appendix F Population Persistence Modeling for Recovery Plan for Tidal Marsh Ecosystems of Northern and Central California with Intial Application to California clapper rail …............................................................................209 Appendix G Glossary……………......................................................................………229 Appendix H Summary of Major Public Comments and Service -

San Francisco Bay Plan
San Francisco Bay Plan San Francisco Bay Conservation and Development Commission In memory of Senator J. Eugene McAteer, a leader in efforts to plan for the conservation of San Francisco Bay and the development of its shoreline. Photo Credits: Michael Bry: Inside front cover, facing Part I, facing Part II Richard Persoff: Facing Part III Rondal Partridge: Facing Part V, Inside back cover Mike Schweizer: Page 34 Port of Oakland: Page 11 Port of San Francisco: Page 68 Commission Staff: Facing Part IV, Page 59 Map Source: Tidal features, salt ponds, and other diked areas, derived from the EcoAtlas Version 1.0bc, 1996, San Francisco Estuary Institute. STATE OF CALIFORNIA GRAY DAVIS, Governor SAN FRANCISCO BAY CONSERVATION AND DEVELOPMENT COMMISSION 50 CALIFORNIA STREET, SUITE 2600 SAN FRANCISCO, CALIFORNIA 94111 PHONE: (415) 352-3600 January 2008 To the Citizens of the San Francisco Bay Region and Friends of San Francisco Bay Everywhere: The San Francisco Bay Plan was completed and adopted by the San Francisco Bay Conservation and Development Commission in 1968 and submitted to the California Legislature and Governor in January 1969. The Bay Plan was prepared by the Commission over a three-year period pursuant to the McAteer-Petris Act of 1965 which established the Commission as a temporary agency to prepare an enforceable plan to guide the future protection and use of San Francisco Bay and its shoreline. In 1969, the Legislature acted upon the Commission’s recommendations in the Bay Plan and revised the McAteer-Petris Act by designating the Commission as the agency responsible for maintaining and carrying out the provisions of the Act and the Bay Plan for the protection of the Bay and its great natural resources and the development of the Bay and shore- line to their highest potential with a minimum of Bay fill. -

(Oncorhynchus Mykiss) in Streams of the San Francisco Estuary, California
Historical Distribution and Current Status of Steelhead/Rainbow Trout (Oncorhynchus mykiss) in Streams of the San Francisco Estuary, California Robert A. Leidy, Environmental Protection Agency, San Francisco, CA Gordon S. Becker, Center for Ecosystem Management and Restoration, Oakland, CA Brett N. Harvey, John Muir Institute of the Environment, University of California, Davis, CA This report should be cited as: Leidy, R.A., G.S. Becker, B.N. Harvey. 2005. Historical distribution and current status of steelhead/rainbow trout (Oncorhynchus mykiss) in streams of the San Francisco Estuary, California. Center for Ecosystem Management and Restoration, Oakland, CA. Center for Ecosystem Management and Restoration TABLE OF CONTENTS Forward p. 3 Introduction p. 5 Methods p. 7 Determining Historical Distribution and Current Status; Information Presented in the Report; Table Headings and Terms Defined; Mapping Methods Contra Costa County p. 13 Marsh Creek Watershed; Mt. Diablo Creek Watershed; Walnut Creek Watershed; Rodeo Creek Watershed; Refugio Creek Watershed; Pinole Creek Watershed; Garrity Creek Watershed; San Pablo Creek Watershed; Wildcat Creek Watershed; Cerrito Creek Watershed Contra Costa County Maps: Historical Status, Current Status p. 39 Alameda County p. 45 Codornices Creek Watershed; Strawberry Creek Watershed; Temescal Creek Watershed; Glen Echo Creek Watershed; Sausal Creek Watershed; Peralta Creek Watershed; Lion Creek Watershed; Arroyo Viejo Watershed; San Leandro Creek Watershed; San Lorenzo Creek Watershed; Alameda Creek Watershed; Laguna Creek (Arroyo de la Laguna) Watershed Alameda County Maps: Historical Status, Current Status p. 91 Santa Clara County p. 97 Coyote Creek Watershed; Guadalupe River Watershed; San Tomas Aquino Creek/Saratoga Creek Watershed; Calabazas Creek Watershed; Stevens Creek Watershed; Permanente Creek Watershed; Adobe Creek Watershed; Matadero Creek/Barron Creek Watershed Santa Clara County Maps: Historical Status, Current Status p. -

West Michigan Pike Route but Is Most Visible Between Whitehall and Shelby
Oceana County Historic Resource Survey 198 Oceana Drive, Rothbury New England Barn & Queen Anne Residence Hart-Montague Trail, Rothbury The trail is twenty-two miles of the former rail bed of the Pere Marquette Railroad. It was made a state park in 1988. The railroad parallels much of the West Michigan Pike route but is most visible between Whitehall and Shelby. New Era New Era was found in 1878 by a group of Dutch that had been living in Montague serving as mill hands. They wanted to return to an agrarian lifestyle and purchased farms and planted peach orchards. In 1947, there were eighty-five Dutch families in New Era. 4856 Oceana, New Era New Era Canning Company The New Era Canning Company was established in 1910 by Edward P. Ray, a Norwegian immigrant who purchased a fruit farm in New Era. Ray grew raspberries, a delicate fruit that is difficult to transport in hot weather. Today, the plant is still owned by the Ray family and processes green beans, apples, and asparagus. Oceana County Historic Resource Survey 199 4775 First Street, New Era New Era Reformed Church 4736 First Street, New Era Veltman Hardware Store Concrete Block Buildings. New Era is characterized by a number of vernacular concrete block buildings. Prior to 1900, concrete was not a common building material for residential or commercial structures. Experimentation, testing and the development of standards for cement and additives in the late 19th century, led to the use of concrete a strong reliable building material after the turn of the century. Concrete was also considered to be fireproof, an important consideration as many communities suffered devastating fires that burned blocks of their wooden buildings Oceana County Historic Resource Survey 200 in the late nineteenth century.