FORDS Manual 2003
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Clinical Practice Guideline for Limb Salvage Or Early Amputation
Limb Salvage or Early Amputation Evidence-Based Clinical Practice Guideline Adopted by: The American Academy of Orthopaedic Surgeons Board of Directors December 6, 2019 Endorsed by: Please cite this guideline as: American Academy of Orthopaedic Surgeons. Limb Salvage or Early Amputation Evidence-Based Clinical Practice Guideline. https://www.aaos.org/globalassets/quality-and-practice-resources/dod/ lsa-cpg-final-draft-12-10-19.pdf Published December 6, 2019 View background material via the LSA CPG eAppendix Disclaimer This clinical practice guideline was developed by a physician volunteer clinical practice guideline development group based on a formal systematic review of the available scientific and clinical information and accepted approaches to treatment and/or diagnosis. This clinical practice guideline is not intended to be a fixed protocol, as some patients may require more or less treatment or different means of diagnosis. Clinical patients may not necessarily be the same as those found in a clinical trial. Patient care and treatment should always be based on a clinician’s independent medical judgment, given the individual patient’s specific clinical circumstances. Disclosure Requirement In accordance with AAOS policy, all individuals whose names appear as authors or contributors to this clinical practice guideline filed a disclosure statement as part of the submission process. All panel members provided full disclosure of potential conflicts of interest prior to voting on the recommendations contained within this clinical practice guideline. Funding Source This clinical practice guideline was funded exclusively through a research grant provided by the United States Department of Defense with no funding from outside commercial sources to support the development of this document. -

Curative Pelvic Exenteration for Recurrent Cervical Carcinoma in the Era of Concurrent Chemotherapy and Radiation Therapy
Available online at www.sciencedirect.com ScienceDirect EJSO xx (2015) 1e11 www.ejso.com Review Curative pelvic exenteration for recurrent cervical carcinoma in the era of concurrent chemotherapy and radiation therapy. A systematic review H. Sardain a,b, V. Lavoue a,b,c,*, M. Redpath d, N. Bertheuil b,e, F. Foucher a,J.Lev^eque a,b,c a CHU de Rennes, Gynecology Department, Tertiary Surgery Center, Teaching Hospital of Rennes, Hopital^ Sud, 16, Bd de Bulgarie, 35000 Rennes, France b Universite de Rennes, Faculty of Medicine, 2 Henry Guilloux, 35000 Rennes, France c INSERM, ER440, Oncogenesis, Stress and Signaling (OSS), Rennes, France d McGill University, Department of Pathology, Jewish General Hospital, Cote^ Sainte Catherine, Montreal, QC, Canada e CHU de Rennes, Department of Plastic, Reconstructive and Aesthetic Surgery, Tertiary Surgery Center, Teaching Hospital of Rennes, Hopital^ Sud, 16, Bd de Bulgarie, 35000 Rennes, France Accepted 26 March 2015 Available online --- Abstract Objective: Pelvic exenteration requires complete resection of the tumor with negative margins to be considered a curative surgery. The pur- pose of this review is to assess the optimal preoperative evaluation and surgical approach in patients with recurrent cervical cancer to in- crease the chances of achieving a curative surgery with decreased morbidity and mortality in the era of concurrent chemoradiotherapy. Methods: Review of English publications pertaining to cervical cancer within the last 25 years were included using PubMed and Cochrane Library searches. Results: Modern imaging (MRI and PET-CT) does not accurately identify local extension of microscopic disease and is inadequate for pre- operative planning of extent of resection. -
Management of Afferent Loop Obstruction: Reoperation Or Endoscopic and Percutaneous Interventions
See discussions, stats, and author profiles for this publication at: https://www.researchgate.net/publication/282163026 Management of afferent loop obstruction: Reoperation or endoscopic and percutaneous interventions Article in World Journal of Gastrointestinal Surgery · September 2015 DOI: 10.4240/wjgs.v7.i9.190 CITATIONS READS 32 427 2 authors, including: Konstantinos Tsalis Aristotle University of Thessaloniki 115 PUBLICATIONS 976 CITATIONS SEE PROFILE Some of the authors of this publication are also working on these related projects: Laparoscopic liver resection using ICG green visualization of hepatic structures View project Laparoscopic liver resection using ICG green visualization of hepatic structures View project All content following this page was uploaded by Konstantinos Tsalis on 26 May 2017. The user has requested enhancement of the downloaded file. Submit a Manuscript: http://www.wjgnet.com/esps/ World J Gastrointest Surg 2015 September 27; 7(9): 190-195 Help Desk: http://www.wjgnet.com/esps/helpdesk.aspx ISSN 1948-9366 (online) DOI: 10.4240/wjgs.v7.i9.190 © 2015 Baishideng Publishing Group Inc. All rights reserved. MINIREVIEWS Management of afferent loop obstruction: Reoperation or endoscopic and percutaneous interventions? Konstantinos Blouhos, Konstantinos Andreas Boulas, Konstantinos Tsalis, Anestis Hatzigeorgiadis Konstantinos Blouhos, Konstantinos Andreas Boulas, Anestis Abstract Hatzigeorgiadis, Department of General Surgery, General Hospital of Drama, 66100 Drama, Greece Afferent loop obstruction is a purely mechanical comp- lication that infrequently occurs following construction Konstantinos Tsalis, D’ Surgical Department, “G. Papanikolaou” of a gastrojejunostomy. The operations most commonly Hospital, Medical School, Aristotle University of Thessaloniki, associated with this complication are gastrectomy 54645 Thessaloniki, Greece with Billroth Ⅱ or Roux-en-Y reconstruction, and pancreaticoduodenectomy with conventional loop or Author contributions: Blouhos K designed the research; Boulas Roux-en-Y reconstruction. -

Pelvic Exenteration for the Management of Pelvic Malignancies
Chapter 7 Pelvic Exenteration for the Management of Pelvic Malignancies Daniel Paramythiotis, Konstantinia Kofina and Antonios Michalopoulos Additional information is available at the end of the chapter http://dx.doi.org/10.5772/61083 Abstract Pelvic exenteration is a surgical procedure first described by Brunschwig in 1948 as a curative or palliative treatment for pelvic and perineal tumors. It is actually a radical operation, involving en bloc resection of pelvic organs, including reproductive structures, bladder, and rectosigmoid. In patients with recurrent cervical and vaginal malignancy, it is associated with a 5-year survival of more than 50%. In spite of advances in surgical management, consequences such as stomas, are still frequently unavoidable for radical tumor excision. Most candidates for this procedure have been diagnosed with recurrent cervical cancer that has previously been treated with surgery and radiation, or radiation alone. Complications of pelvic exenteration are more severe than those of standard resection of a colorectal carcinoma, so it is not commonly performed, including wound infection, wound dehiscence (also described as burst abdomen) the creation of fistulae (perineal-fecal, uretero-vaginal, between conduit and perineal wound), urinary tract infections, perineal hernias and intestinal obstruction. Patients need to be carefully selected and counseled about risks and long-term issues related to the surgery. A comprehensive evaluation is required in order to exclude unresectable or metastatic disease. Evolution of the technique through laparoscopy and minimally invasive surgery may result in a reduction of morbidity and mortality. Keywords: Pelvic exenteration, gynecologic cancer 1. Introduction Pelvic exenteration was first described by Brunschwig and his colleagues of New York’s Memorial Hospital in 1948 [1] and was initially performed as a palliative surgical intervention © 2015 The Author(s). -

Treating Cervical Cancer If You've Been Diagnosed with Cervical Cancer, Your Cancer Care Team Will Talk with You About Treatment Options
cancer.org | 1.800.227.2345 Treating Cervical Cancer If you've been diagnosed with cervical cancer, your cancer care team will talk with you about treatment options. In choosing your treatment plan, you and your cancer care team will also take into account your age, your overall health, and your personal preferences. How is cervical cancer treated? Common types of treatments for cervical cancer include: ● Surgery for Cervical Cancer ● Radiation Therapy for Cervical Cancer ● Chemotherapy for Cervical Cancer ● Targeted Therapy for Cervical Cancer ● Immunotherapy for Cervical Cancer Common treatment approaches Depending on the type and stage of your cancer, you may need more than one type of treatment. For the earliest stages of cervical cancer, either surgery or radiation combined with chemo may be used. For later stages, radiation combined with chemo is usually the main treatment. Chemo (by itself) is often used to treat advanced cervical cancer. ● Treatment Options for Cervical Cancer, by Stage Who treats cervical cancer? Doctors on your cancer treatment team may include: 1 ____________________________________________________________________________________American Cancer Society cancer.org | 1.800.227.2345 ● A gynecologist: a doctor who treats diseases of the female reproductive system ● A gynecologic oncologist: a doctor who specializes in cancers of the female reproductive system who can perform surgery and prescribe chemotherapy and other medicines ● A radiation oncologist: a doctor who uses radiation to treat cancer ● A medical oncologist: a doctor who uses chemotherapy and other medicines to treat cancer Many other specialists may be involved in your care as well, including nurse practitioners, nurses, psychologists, social workers, rehabilitation specialists, and other health professionals. -

En Bloc Resection of Extra-Peritoneal Soft Tissue Neoplasms Incorporating a Type III Internal Hemipelvectomy: a Novel Approach Sanjay S Reddy1* and Norman D Bloom2
Reddy and Bloom World Journal of Surgical Oncology 2012, 10:222 http://www.wjso.com/content/10/1/222 WORLD JOURNAL OF SURGICAL ONCOLOGY REVIEW Open Access En bloc resection of extra-peritoneal soft tissue neoplasms incorporating a type III internal hemipelvectomy: a novel approach Sanjay S Reddy1* and Norman D Bloom2 Abstract Background: A type III hemipelvectomy has been utilized for the resection of tumors arising from the superior or inferior pubic rami. Methods: In eight patients, we incorporated a type III internal hemipelvectomy to achieve an en bloc R0 resection for tumors extending through the obturator foramen or into the ischiorectal fossa. The pelvic ring was reconstructed utilizing marlex mesh. This allowed for pelvic stability and abdominal wall reconstruction with obliteration of the obturator space to prevent herniations. Results: All eight patients had an R0 resection with an overall survival of 88% and with average follow up of 9.5 years. Functional evaluation utilizing the Enneking classification system, which evaluates motion, pain, stability and strength of the affected extremity, revealed a 62% excellent result and a 37% good result. No significant complications were associated with the operative procedure. Marlex mesh reconstruction provided pelvic stability and eliminated all hernial defects. Conclusion: The superior and inferior pubic rami provide a barrier to a resection for tumors that arise in the extra-peritoneal pelvis extending through the obturator foramen or ischiorectal fossa. Incorporating a type III internal -

Gender Confirmation Surgery Reference Number: PA.CP.MP.95 Effective Date: 01/18 Coding Implications Last Review Date: 09/17 Revision Log
Clinical Policy: Gender Confirmation Surgery Reference Number: PA.CP.MP.95 Effective Date: 01/18 Coding Implications Last Review Date: 09/17 Revision Log Description Services for gender confirmation most often include hormone treatment, counseling, psychotherapy, complete hysterectomy, bilateral mastectomy, chest reconstruction or augmentation as appropriate, genital reconstruction, facial hair removal, and certain facial plastic reconstruction. Not every individual will require each intervention so necessity needs to be considered on an individualized basis. This criteria outlines medical necessity criteria for gender confirmation surgery when such services are included under the members’ benefit plan contract provisions. Policy/Criteria It is the policy of Pennsylvania Health and Wellness® (PHW) that the gender confirmation surgeries listed in section III are considered medically necessary for members when diagnosed with gender dysphoria per criteria in section I and when meeting eligibility criteria in section II. I. Gender Dysphoria Criteria, meets A and B A. Marked incongruence between the member’s experienced/expressed gender and assigned gender, of at least 6 month’s duration, as indicated by two or more of the following: 1. Marked incongruence between the member’s experienced/expressed gender and primary and/or secondary sex characteristics; 2. A strong desire to be rid of one’s primary and/or secondary sex characteristics because of a marked incongruence with one’s experienced/expressed gender; 3. A strong desire for the primary and/or secondary sex characteristics of the other gender; 4. A strong desire to be of the other gender (or some alternative gender different from one’s assigned gender); 5. A strong desire to be treated as the other gender (or some alternative gender different from one’s assigned gender); 6. -
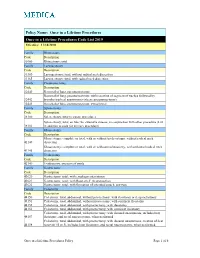
Once in a Lifetime Procedures Code List 2019 Effective: 11/14/2010
Policy Name: Once in a Lifetime Procedures Once in a Lifetime Procedures Code List 2019 Effective: 11/14/2010 Family Rhinectomy Code Description 30160 Rhinectomy; total Family Laryngectomy Code Description 31360 Laryngectomy; total, without radical neck dissection 31365 Laryngectomy; total, with radical neck dissection Family Pneumonectomy Code Description 32440 Removal of lung, pneumonectomy; Removal of lung, pneumonectomy; with resection of segment of trachea followed by 32442 broncho-tracheal anastomosis (sleeve pneumonectomy) 32445 Removal of lung, pneumonectomy; extrapleural Family Splenectomy Code Description 38100 Splenectomy; total (separate procedure) Splenectomy; total, en bloc for extensive disease, in conjunction with other procedure (List 38102 in addition to code for primary procedure) Family Glossectomy Code Description Glossectomy; complete or total, with or without tracheostomy, without radical neck 41140 dissection Glossectomy; complete or total, with or without tracheostomy, with unilateral radical neck 41145 dissection Family Uvulectomy Code Description 42140 Uvulectomy, excision of uvula Family Gastrectomy Code Description 43620 Gastrectomy, total; with esophagoenterostomy 43621 Gastrectomy, total; with Roux-en-Y reconstruction 43622 Gastrectomy, total; with formation of intestinal pouch, any type Family Colectomy Code Description 44150 Colectomy, total, abdominal, without proctectomy; with ileostomy or ileoproctostomy 44151 Colectomy, total, abdominal, without proctectomy; with continent ileostomy 44155 Colectomy, -

Use of Anterolateral Thigh Flap for Reconstruction of Traumatic Bilateral Hemipelvectomy After Major Pelvic Trauma
Al‑wageeh et al. surg case rep (2020) 6:247 https://doi.org/10.1186/s40792‑020‑01009‑2 CASE REPORT Open Access Use of anterolateral thigh fap for reconstruction of traumatic bilateral hemipelvectomy after major pelvic trauma: a case report Saleh Al‑wageeh1 , Faisal Ahmed2* , Khalil Al‑naggar3 , Mohammad Reza Askarpour4 and Ebrahim Al‑shami5 Abstract Background: Major pelvic trauma (MPT) with traumatic hemipelvectomy (THP) is rare, but it is a catastrophic health problem caused by high‑energy injury leading to separation of the lower extremity from the axial skeleton, which is associated with a high incidence of intra‑abdominal and multi‑systemic injuries. THP is generally performed as a lifesaving protocol to return the patient to an active life. Case report: A 12‑year male patient exposed to major pelvic trauma with bilateral THP survived the trauma and mul‑ tiple lifesaving operations. The anterolateral thigh fap is the method used for wound reconstruction. The follow‑up was ended with colostomy and cystostomy with wheelchair mobilization. To the best of our knowledge, there have been a few bilateral THP reports, and our case is the second one to be successfully treated with an anterolateral thigh fap. Conclusion: MPT with THP is the primary cause of death among trauma patients. Life‑threatening hemorrhage is the usual cause of death, which is a strong indication for THP to save life. Keywords: Amputation, Hemipelvectomy, Myocutaneous fap, Reconstruction, Trauma Introduction A few victims survive these injuries, and the actual Major pelvic trauma (MPT) associated with traumatic incidence is unknown, but it is usually underestimated hemipelvectomy (THP) was described frst by Turnbull in [3]. -

Pelvic Exenteration As Ultimate Ratio for Gynecologic Cancers: Single‑Center Analyses of 37 Cases
Archives of Gynecology and Obstetrics (2019) 300:161–168 https://doi.org/10.1007/s00404-019-05154-4 GYNECOLOGIC ONCOLOGY Pelvic exenteration as ultimate ratio for gynecologic cancers: single‑center analyses of 37 cases N. de Gregorio1 · A. de Gregorio1 · F. Ebner2 · T. W. P. Friedl1 · J. Huober1 · R. Hefty3 · M. Wittau4 · W. Janni1 · P. Widschwendter1 Received: 5 February 2019 / Accepted: 5 April 2019 / Published online: 22 April 2019 © Springer-Verlag GmbH Germany, part of Springer Nature 2019 Abstract Background Pelvic exenterations are a last resort procedure for advanced gynecologic malignancies with elevated risks in terms of patients’ morbidity. Methods This single-center analysis reports surgical details, outcome and survival of all patients treated with exenteration for non-ovarian gynecologic malignancies at our university hospital during a 13-year time period. We collected data regarding patients and tumor characteristics, surgical procedures, peri- and postoperative management, transfusions, complications, and analyzed the impact on survival outcomes. Results We identifed 37 patients between 2005 and 2013 with primary or relapsed cervical cancer (59.5%), vulvar cancer (24.3%) or endometrial cancer (16.2%). Median age was 60 years and most patients (73%) had squamous cell carcinomas. Median progression-free survival was 26.2 months and median overall survival was 49.9 months. The 5-year survival rates were 34.4% for progression-free survival and 46.4% for overall survival. There were no signifcant diferences in progres- sion-free survival and overall survival with regard to disease entity. Patients with tumor at the resection margins (R1) had a nearly signifcantly worse progression-free survival (median: 28.5 vs. -

Seer Program Code Manual
THE SEER PROGRAM CODE MANUAL Revised Edition June 1992 CANCER STATISTICS BRANCH SURVEILLANCE PROGRAM DIVISION OF CANCER PREVENTION AND CONTROL NATIONAL CANCER INSTITUTE U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES PUBLIC HEALTH SERVICE NATIONAL INSTITUTES OF HEALTH Effective Date: Cases Diagnosed January 1, 1992 The SEER Program Code Manual Revised Edition June 1992 Editors Jack Cunningham Lynn Ries Benjamin Hankey Jennifer Seiffert Barbara Lyles Evelyn Shambaugh Constance Percy Valerie Van Holten Acknowledgements The editors wish to acknowledge the assistance of Terry Swenson, Maureen Troublefield, Diane Licitra, and Jerome Felix of Information Management Services, Inc., in the preparation of the SEER Program Code Manual. The editors also wish to acknowledge the assistance of Dr. John Berg in preparation of the section on multiple primary determination for lymphatic and hematopoietic diseases. TABLE OF CONTENTS PREFACE TO THE REVISED EDITION ....................................... vii COMPUTER RECORD FORMAT ............................................ 1 INTRODUCTION AND GENERAL INSTRUCTIONS .............................. 5 REFERENCES .......................................................... 37 SEER CODE SUMMARY .................................................. 39 I BASIC RECORD IDENTIFICATION ...................................... 57 1.01 SEER Participant ........................................... 58 1.02 Case Number .............................................. 59 1.03 Record Number ............................................ 60 -

Safe, Simple & Efficient Totally Laparoscopic Billroth II Gastrectomy
China Gastric Cancer Research Highlight Safe, simple & efficient totally laparoscopic Billroth II gastrectomy by only stapling devices Kirubakaran Malapan1, Chih-Kun Huang1,2 1Department of Bariatric and Metabolic International Surgery Centre, E-Da Hospital, Kaohsiung City, 82445, Taiwan; 2The First Affiliated Hospital of Guangzhou Medical University, Guangzhou 510120, China Corresponding to: Chih-Kun Huang, M.D., Director. Department of Bariatric and Metabolic International Surgery Centre, E-da Hospital, No 1, E-Da Rd., Yan-chau District, Kaohsiung City, Taiwan, 82445. Email: [email protected]. Submitted May 08, 2013. Accepted for publication May 28, 2013. doi: 10.3978/j.issn.2224-4778.2013.05.28 Scan to your mobile device or view this article at: http://www.amepc.org/tgc/article/view/2081/2870 Gastric cancer is the fourth most common cancer diagnosis combination of both. Du J et al. reported their experience worldwide in men with an expected incidence of 640,000 of intracorporeal gastrojejunal anastomosis using a two cases and the fifth most common in women with an expected layer hand-sewn technique (9), whereas Ruiz et al. described incidence of 350,000 cases in 2011 (1). Approximately, 8% a 4-layer closure using continuous absorbable sutures (10). of total cases and 10% of annual cancer deaths worldwide Hand-sewn anastomosis requires advanced laparoscopic are attributed to this dreaded disease. Surgical resection skills and is considered to be time-consuming, but has offers the only durable cure from gastric cancer (2). Since the advantage of avoiding the risk of wound infection and the introduction of Billroth’s procedure of gastrectomy and hernias, which occur as a result of manipulation by a circular reconstruction in 1881, surgical techniques in gastric surgery stapler.