Accuracy of Colposcopy-Directed Biopsy in Detecting Early Cervical
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Gender Confirmation Surgery Reference Number: PA.CP.MP.95 Effective Date: 01/18 Coding Implications Last Review Date: 09/17 Revision Log
Clinical Policy: Gender Confirmation Surgery Reference Number: PA.CP.MP.95 Effective Date: 01/18 Coding Implications Last Review Date: 09/17 Revision Log Description Services for gender confirmation most often include hormone treatment, counseling, psychotherapy, complete hysterectomy, bilateral mastectomy, chest reconstruction or augmentation as appropriate, genital reconstruction, facial hair removal, and certain facial plastic reconstruction. Not every individual will require each intervention so necessity needs to be considered on an individualized basis. This criteria outlines medical necessity criteria for gender confirmation surgery when such services are included under the members’ benefit plan contract provisions. Policy/Criteria It is the policy of Pennsylvania Health and Wellness® (PHW) that the gender confirmation surgeries listed in section III are considered medically necessary for members when diagnosed with gender dysphoria per criteria in section I and when meeting eligibility criteria in section II. I. Gender Dysphoria Criteria, meets A and B A. Marked incongruence between the member’s experienced/expressed gender and assigned gender, of at least 6 month’s duration, as indicated by two or more of the following: 1. Marked incongruence between the member’s experienced/expressed gender and primary and/or secondary sex characteristics; 2. A strong desire to be rid of one’s primary and/or secondary sex characteristics because of a marked incongruence with one’s experienced/expressed gender; 3. A strong desire for the primary and/or secondary sex characteristics of the other gender; 4. A strong desire to be of the other gender (or some alternative gender different from one’s assigned gender); 5. A strong desire to be treated as the other gender (or some alternative gender different from one’s assigned gender); 6. -
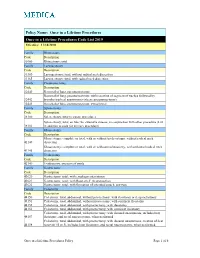
Once in a Lifetime Procedures Code List 2019 Effective: 11/14/2010
Policy Name: Once in a Lifetime Procedures Once in a Lifetime Procedures Code List 2019 Effective: 11/14/2010 Family Rhinectomy Code Description 30160 Rhinectomy; total Family Laryngectomy Code Description 31360 Laryngectomy; total, without radical neck dissection 31365 Laryngectomy; total, with radical neck dissection Family Pneumonectomy Code Description 32440 Removal of lung, pneumonectomy; Removal of lung, pneumonectomy; with resection of segment of trachea followed by 32442 broncho-tracheal anastomosis (sleeve pneumonectomy) 32445 Removal of lung, pneumonectomy; extrapleural Family Splenectomy Code Description 38100 Splenectomy; total (separate procedure) Splenectomy; total, en bloc for extensive disease, in conjunction with other procedure (List 38102 in addition to code for primary procedure) Family Glossectomy Code Description Glossectomy; complete or total, with or without tracheostomy, without radical neck 41140 dissection Glossectomy; complete or total, with or without tracheostomy, with unilateral radical neck 41145 dissection Family Uvulectomy Code Description 42140 Uvulectomy, excision of uvula Family Gastrectomy Code Description 43620 Gastrectomy, total; with esophagoenterostomy 43621 Gastrectomy, total; with Roux-en-Y reconstruction 43622 Gastrectomy, total; with formation of intestinal pouch, any type Family Colectomy Code Description 44150 Colectomy, total, abdominal, without proctectomy; with ileostomy or ileoproctostomy 44151 Colectomy, total, abdominal, without proctectomy; with continent ileostomy 44155 Colectomy, -

Vaginal Hysterectomy: Carl W
OBGM_0306_Zimmerman.finalREV 2/22/06 2:51 PM Page 21 SURGICALTECHNIQUES Vaginal hysterectomy: Carl W. Zimmerman, MD Professor of Obstetrics and Gynecology, Vanderbilt University Is skill the limiting factor? School of Medicine, Nashville, Tenn For the expert surgeon, there is no absolute uterine size limit CASE Bleeding, a large uterus, aginal hysterectomy is not only fea- and no response to hormones sible, it is preferred. Although V laparoscopic surgeons are fond of “M.G.,” a 42-year-old nullipara, complains using the phrase “minimally invasive sur- of menstrual periods that last 10 days and gery” to describe their procedures, when it occur on a 28-day cycle. She says the ® comesDowden to hysterectomy, Health only Media the vaginal bleeding is extremely heavy, with frequent, route qualifies for this superlative descrip- copious clotting. She routinely avoids tion. And although uterine size does some- planning social activities aroundCopyright the timeFor timespersonal limit use ofuse the vaginal only route, it need of her period and occasionally cancels do so in only a minority of cases. nonessential engagements because of it. This article describes surgical tech- Over the past year, this professional niques for vaginal removal of the large woman has missed 6 days of work uterus, using morcellation, coring, cervi- IN THIS ARTICLE because of the problem with her menses. cectomy, and other strategies. When you ask about her history, she ❙ How to choose reports that another gynecologist first Is the vaginal approach always best? a hysterectomy palpated an enlarged and irregular uterus Guidelines addressing this question were route 5 years earlier, and an ultrasound at that developed by the Society of Pelvic Page 22 time revealed a multinodular fundus of Reconstructive Surgeons and evaluated by approximately 12 weeks’ size. -

Cervix Uteri Surgery Codes
SEER Program Coding and Staging Manual 2015 Cervix Uteri C530–C539 (Except for M9727, 9733, 9741-9742, 9764-9809, 9832, 9840-9931, 9945-9946, 9950-9967, 9975-9992) [SEER Note: Do not code dilation and curettage (D&C) as Surgery of Primary Site for invasive cancers] Codes 00 None; no surgery of primary site; autopsy ONLY 10 Local tumor destruction, NOS 11 Photodynamic therapy (PDT) 12 Electrocautery; fulguration (includes use of hot forceps for tumor destruction) 13 Cryosurgery 14 Laser 15 Loop Electrocautery Excision Procedure (LEEP) 16 Laser ablation 17 Thermal ablation No specimen sent to pathology from surgical events 10–17 20 Local tumor excision, NOS [SEER Note: Margins of resection may have microscopic involvement. Procedures in code 20 include but are not limited to: cryosurgery, electrocautery, excisional biopsy, laser ablation, or thermal ablation.] 26 Excisional biopsy, NOS 27 Cone biopsy 24 Cone biopsy WITH gross excision of lesion 29 Trachelectomy; removal of cervical stump; cervicectomy Any combination of 20, 24, 26, 27 or 29 WITH 21 Electrocautery 22 Cryosurgery 23 Laser ablation or excision 25 Dilatation and curettage; endocervical curettage (for in situ only) 28 Loop electrocautery excision procedure (LEEP) 30 Total hysterectomy (simple, pan-) WITHOUT removal of tubes and ovaries Total hysterectomy removes both the corpus and the cervix uteri and may also include a portion of vaginal cuff 40 Total hysterectomy (simple, pan-) WITH removal of tubes and/or ovary Total hysterectomy removes both the corpus and the cervix uteri -

OBGYN Outpatient Surgery Coding
OBGYN Outpatient Surgery Coding Anatomy Anatomy • Hyster/o – uterus, womb • Uter/o – uterus, womb • Metr/o – uterus, womb • Salping/o – tube, usually fallopian tube • Oophor/o – ovary • Ovari/o - ovary Terminology • Colpo – vagina • Cervic/o – cervix, lower part of the uterus, the “neck” • Episi/o – vulva • Vulv/o – vulva • Perine/o – the space between the anus and vulva Hysterectomy • A hysterectomy is an operation to remove a woman's uterus. • A woman may have a hysterectomy for different reasons, including: • Uterine fibroids that cause pain • bleeding, or other problems. • Uterine prolapse, which is a sliding of the uterus from its normal position into the vaginal canal. Hysterectomy • There are around 30 hysterectomy CPT codes. • To find the correct code you have to first check: • the surgical approach and • extent of the procedure. Surgical Approaches • Abdominal – the uterus is removed via an incision in the lower abdomen • Vaginal – the uterus is removed via an incision in the vagina • Laparoscopic – the procedure is performed using a laparoscope , inserted via several small incisions in the body. • Their are also CPT codes for laparoscopic-assisted vaginal approach. In this procedure ,the scope is inserted via a small incisions in the vagina. Extent of Procedure • Total hysterectomy: It includes laparoscopically detaching the entire uterine cervix and body from the surrounding supporting structures and suturing the vaginal cuff. It includes bivalving, coring, or morcellating the excised tissues, as required. The uterus is then removed through the vagina or abdomen. • Subtotal, partial or supracervical hysterectomy: It is the removal of the fundus or op portion of the uterus only, leaving the cervix in place. -

Complete Draft Code Set Table of Contents
Draft 2012 Procedural Coding System ICD~10~PCS Complete Draft Code Set Table of Contents Introduction . 1 Eye 080–08Y . 75 The ICD-10 Procedure Coding System (ICD-10-PCS) . 1 Ear Nose and Sinus 090–09W . 86 Introduction . 1 Respiratory System 0B1–0BY . 99 General Development Principles . 1 Mouth and Throat 0C0–0CX . 112 ICD-10-PCS Structure . .. 1 Gastrointestinal System 0D1–0DY . 123 ICD-10-PCS Format . 1 Hepatobiliary System and Pancreas 0F1–0FY . 141 Medical and Surgical Section (0) . 2 Endocrine System 0G2–0GW . 150 Obstetrics Section . 5 Skin and Breast 0H0–0HY . 155 Placement Section . 5 Subcutaneous Tissue and Fascia 0J0–0JX . .166 Administration Section . 6 Muscles 0K2–0KX . 181 Measurement and Monitoring Section . 6 Tendons 0L2–0LX . 189 Extracorporeal Assistance and Performance Section . 7 Bursae and Ligaments 0M2–0MX . .197 Extracorporeal Therapies Section . 7 Head and Facial Bones 0N2–0NW . 207 Osteopathic Section . 7 Upper Bones 0P2–0PW . 217 Other Procedures Section . 8 Lower Bones 0Q2–0QW . 227 Chiropractic Section . 8 Upper Joints 0R2–0RW . 237 Imaging Section . 8 Lower Joints 0S2–0SW . 249 Nuclear Medicine Section . 9 Urinary System 0T1–0TY . 262 Radiation Oncology Section . 9 Female Reproductive System 0U1–0UY . 272 Physical Rehabilitation and Diagnostic Audiology Male Reproductive System 0V1–0VW . 284 Section . 10 Anatomical Regions, General 0W0–0WW . 295 Mental Health Section . 10 Anatomical Regions, Upper Extremities 0X0–0XX . 303 Substance Abuse Treatment Section . 10 Anatomical Regions, Lower Extremities 0Y0–0YR . 309 Modifications to ICD-10-PCS . 10 Obstetrics 102–10Y . 315 Number of Codes in ICD-10-PCS . 11 Placement, Anatomical Regions 2W0–2W6 . -
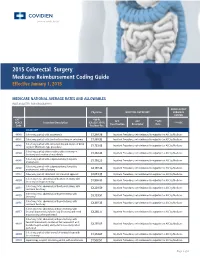
2015 Colorectal Surgery Medicare Reimbursement Coding Guide Effective January 1, 2015
2015 Colorectal Surgery Medicare Reimbursement Coding Guide Effective January 1, 2015 MEDICARE NATIONAL AVERAGE RATES AND ALLOWABLES (NOT ADJUSTED FOR GEOGRAPHY) AMBULATORY Physician HOSPITAL OUPATIENT SURGICAL CENTER CPT™* *MPFS APC APC **APC HCPCS Procedure Description (CF=$35.7547) ***ASC Classification Descriptor Rate Code Fac/Non-Fac COLECTOMY 44140 Colectomy, partial; with anastomosis $1,388.36 Inpatient Procedures, not reimbursed in outpatient or ASC by Medicare 44141 Colectomy, partial; with skin level cecostomy or colostomy $1,888.92 Inpatient Procedures, not reimbursed in outpatient or ASC by Medicare Colectomy, partial; with end colostomy and closure of distal 44143 Inpatient Procedures, not reimbursed in outpatient or ASC by Medicare segment (Hartmann type procedure) $1,723.02 Colectomy, partial; with resection, with colostomy or 44144 Inpatient Procedures, not reimbursed in outpatient or ASC by Medicare ileostomy and creation of mucofistula $1,832.43 Colectomy, partial; with coloproctostomy (low pelvic 44145 Inpatient Procedures, not reimbursed in outpatient or ASC by Medicare anastomosis) $1,716.23 Colectomy, partial; with coloproctostomy (low pelvic 44146 Inpatient Procedures, not reimbursed in outpatient or ASC by Medicare anastomosis), with colostomy $2,197.84 44147 Colectomy, partial; abdominal and transanal approach $2,013.35 Inpatient Procedures, not reimbursed in outpatient or ASC by Medicare Colectomy, total, abdominal, without proctectomy; with 44150 Inpatient Procedures, not reimbursed in outpatient or ASC by Medicare -

SJH Procedures
SJH Procedures - Gynecology and Gynecology Oncology Services New Name Old Name CPT Code Service ABLATION, LESION, CERVIX AND VULVA, USING CO2 LASER LASER VAPORIZATION CERVIX/VULVA W CO2 LASER 56501 Destruction of lesion(s), vulva; simple (eg, laser surgery, Gynecology electrosurgery, cryosurgery, chemosurgery) 56515 Destruction of lesion(s), vulva; extensive (eg, laser surgery, Gynecology electrosurgery, cryosurgery, chemosurgery) 57513 Cautery of cervix; laser ablation Gynecology BIOPSY OR EXCISION, LESION, FACE AND NECK EXCISION/BIOPSY (MASS/LESION/LIPOMA/CYST) FACE/NECK General, Gynecology, Plastics, ENT, Maxillofacial BIOPSY OR EXCISION, LESION, FACE AND NECK, 2 OR MORE EXCISE/BIOPSY (MASS/LESION/LIPOMA/CYST) MULTIPLE FACE/NECK 11102 Tangential biopsy of skin (eg, shave, scoop, saucerize, curette); General, Gynecology, single lesion Aesthetics, Urology, Maxillofacial, ENT, Thoracic, Vascular, Cardiovascular, Plastics, Orthopedics 11103 Tangential biopsy of skin (eg, shave, scoop, saucerize, curette); General, Gynecology, each separate/additional lesion (list separately in addition to Aesthetics, Urology, code for primary procedure) Maxillofacial, ENT, Thoracic, Vascular, Cardiovascular, Plastics, Orthopedics 11104 Punch biopsy of skin (including simple closure, when General, Gynecology, performed); single lesion Aesthetics, Urology, Maxillofacial, ENT, Thoracic, Vascular, Cardiovascular, Plastics, Orthopedics 11105 Punch biopsy of skin (including simple closure, when General, Gynecology, performed); each separate/additional lesion -

Outpatient Surgical Procedures – Site of Service: CPT/HCPCS Codes
UnitedHealthcare® Commercial Policy Appendix: Applicable Code List Outpatient Surgical Procedures – Site of Service: CPT/HCPCS Codes This list of codes applies to the Utilization Review Guideline titled Effective Date: August 1, 2021 Outpatient Surgical Procedures – Site of Service. Applicable Codes The following list(s) of procedure and/or diagnosis codes is provided for reference purposes only and may not be all inclusive. The listing of a code does not imply that the service described by the code is a covered or non-covered health service. Benefit coverage for health services is determined by the member specific benefit plan document and applicable laws that may require coverage for a specific service. The inclusion of a code does not imply any right to reimbursement or guarantee claim payment. Other Policies and Guidelines may apply. This list contains CPT/HCPCS codes for the following: • Auditory System • Female Genital System • Musculoskeletal System • Cardiovascular System • Hemic and Lymphatic Systems • Nervous System • Digestive System • Integumentary System • Respiratory System • Eye/Ocular Adnexa System • Male Genital System • Urinary System CPT Code Description Auditory System 69100 Biopsy external ear 69110 Excision external ear; partial, simple repair 69140 Excision exostosis(es), external auditory canal 69145 Excision soft tissue lesion, external auditory canal 69205 Removal foreign body from external auditory canal; with general anesthesia 69222 Debridement, mastoidectomy cavity, complex (e.g., with anesthesia or more -

Bt201229 July 31, 2012
IHCP bulletin INDIANA HEALTH COVERAGE PROGRAMS BT201229 JULY 31, 2012 Coverage of medically necessary hysterectomies requires proper documentation Per 405 IAC 5-28-9, the Indiana Health Coverage Programs (IHCP) provides coverage for a medically necessary hyster- ectomy performed to treat an illness or injury only when the member has given informed consent and prior authorization (PA) has been obtained. Effective for dates of service on or after September 1, 2012, the procedure codes in the table on the next page will be added to the list of hysterectomy codes requiring PA. Continue IHCP bulletin BT201229 JULY 31, 2012 Hysterectomy codes requiring PA effective for dates of service on or after September 1, 2012 Procedure code Description Pelvic exenteration for colorectal malignancy, with proctectomy [with or without colostomy], with 45126 removal of bladder and ureteral transplantations, and/or hysterectomy, or cervicectomy, with or without removal of tube[s], with or without removal of ovary[s], or any combination thereof 51925 Closure of vesicouterine fistula; with hysterectomy Pelvic exenteration, complete, for vesical, prostatic or urethral malignancy, with removal of blad- 51597 der and ureteral transplantations, with or without hysterectomy and/or abdominoperineal resection of rectum and colon and colostomy, or any combination thereof Radical abdominal hysterectomy, with bilateral total pelvic lymphadenectomy and para-aortic 58210 lymph node sampling (biopsy), with or without removal of tube(s), with or without removal of ovary(s) -
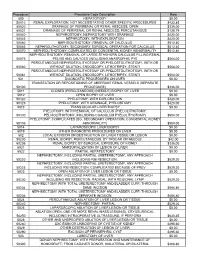
Procedure Procedure Code Description Rate 500
Procedure Procedure Code Description Rate 500 HEPATOTOMY $0.00 50010 RENAL EXPLORATION, NOT NECESSITATING OTHER SPECIFIC PROCEDURES $433.85 50020 DRAINAGE OF PERIRENAL OR RENAL ABSCESS; OPEN $336.00 50021 DRAINAGE OF PERIRENAL OR RENAL ABSCESS; PERCUTANIOUS $128.79 50040 NEPHROSTOMY, NEPHROTOMY WITH DRAINAGE $420.00 50045 NEPHROTOMY, WITH EXPLORATION $420.00 50060 NEPHROLITHOTOMY; REMOVAL OF CALCULUS $512.40 50065 NEPHROLITHOTOMY; SECONDARY SURGICAL OPERATION FOR CALCULUS $512.40 50070 NEPHROLITHOTOMY; COMPLICATED BY CONGENITAL KIDNEY ABNORMALITY $512.40 NEPHROLITHOTOMY; REMOVAL OF LARGE STAGHORN CALCULUS FILLING RENAL 50075 PELVIS AND CALYCES (INCLUDING ANATROPHIC PYE $504.00 PERCUTANEOUS NEPHROSTOLITHOTOMY OR PYELOSTOLITHOTOMY, WITH OR 50080 WITHOUT DILATION, ENDOSCOPY, LITHOTRIPSY, STENTI $504.00 PERCUTANEOUS NEPHROSTOLITHOTOMY OR PYELOSTOLITHOTOMY, WITH OR 50081 WITHOUT DILATION, ENDOSCOPY, LITHOTRIPSY, STENTI $504.00 501 DIAGNOSTIC PROCEDURES ON LIVER $0.00 TRANSECTION OR REPOSITIONING OF ABERRANT RENAL VESSELS (SEPARATE 50100 PROCEDURE) $336.00 5011 CLOSED (PERCUTANEOUS) (NEEDLE) BIOPSY OF LIVER $0.00 5012 OPEN BIOPSY OF LIVER $0.00 50120 PYELOTOMY; WITH EXPLORATION $420.00 50125 PYELOTOMY; WITH DRAINAGE, PYELOSTOMY $420.00 5013 TRANSJUGULAR LIVER BIOPSY $0.00 PYELOTOMY; WITH REMOVAL OF CALCULUS (PYELOLITHOTOMY, 50130 PELVIOLITHOTOMY, INCLUDING COAGULUM PYELOLITHOTOMY) $504.00 PYELOTOMY; COMPLICATED (EG, SECONDARY OPERATION, CONGENITAL KIDNEY 50135 ABNORMALITY) $504.00 5014 LAPAROSCOPIC LIVER BIOPSY $0.00 5019 OTHER DIAGNOSTIC PROCEDURES -
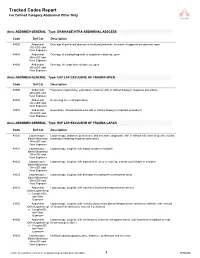
Tracked Codes Report for Defined Category Abdominal Other Only
Tracked Codes Report For Defined Category Abdominal Other Only Area: ABDOMEN-GENERAL Type: DRAINAGE INTRA-ABDOMINAL ABSCESS Code Def Cat Description 49020 Abdominal Drainage of peritoneal abscess or localized peritonitis, exclusive of appendiceal abscess, open Other/DC and Vasc Exposure 49040 Abdominal Drainage of subdiaphragmatic or subphrenic abscess, open Other/DC and Vasc Exposure 49060 Abdominal Drainage of retroperitoneal abscess, open Other/DC and Vasc Exposure Area: ABDOMEN-GENERAL Type: EXP LAP EXCLUSIVE OF TRAUMA-OPEN Code Def Cat Description 49000 Abdominal Exploratory laparotomy, exploratory celiotomy with or without biopsy(s) (separate procedure) Other/DC and Vasc Exposure 49002 Abdominal Reopening of recent laparotomy Other/DC and Vasc Exposure 49010 Abdominal Exploration, retroperitoneal area with or without biopsy(s) (separate procedure) Other/DC and Vasc Exposure Area: ABDOMEN-GENERAL Type: EXP LAP EXCLUSIVE OF TRAUMA-LAPAR Code Def Cat Description 49320 Laparoscopic - Laparoscopy, abdomen, peritoneum, and omentum, diagnostic, with or without collection of specimen(s) by Basic/Abdominal brushing or washing (separate procedure) Other/DC and Vasc Exposure 49321 Laparoscopic - Laparoscopy, surgical; with biopsy (single or multiple) Basic/Abdominal Other/DC and Vasc Exposure 49322 Laparoscopic - Laparoscopy, surgical; with aspiration of cavity or cyst (eg, ovarian cyst) (single or multiple) Basic/Abdominal Other/DC and Vasc Exposure 49323 Laparoscopic - Laparoscopy, surgical; with drainage of lymphocele to peritoneal cavity