Seer Program Code Manual
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Curative Pelvic Exenteration for Recurrent Cervical Carcinoma in the Era of Concurrent Chemotherapy and Radiation Therapy
Available online at www.sciencedirect.com ScienceDirect EJSO xx (2015) 1e11 www.ejso.com Review Curative pelvic exenteration for recurrent cervical carcinoma in the era of concurrent chemotherapy and radiation therapy. A systematic review H. Sardain a,b, V. Lavoue a,b,c,*, M. Redpath d, N. Bertheuil b,e, F. Foucher a,J.Lev^eque a,b,c a CHU de Rennes, Gynecology Department, Tertiary Surgery Center, Teaching Hospital of Rennes, Hopital^ Sud, 16, Bd de Bulgarie, 35000 Rennes, France b Universite de Rennes, Faculty of Medicine, 2 Henry Guilloux, 35000 Rennes, France c INSERM, ER440, Oncogenesis, Stress and Signaling (OSS), Rennes, France d McGill University, Department of Pathology, Jewish General Hospital, Cote^ Sainte Catherine, Montreal, QC, Canada e CHU de Rennes, Department of Plastic, Reconstructive and Aesthetic Surgery, Tertiary Surgery Center, Teaching Hospital of Rennes, Hopital^ Sud, 16, Bd de Bulgarie, 35000 Rennes, France Accepted 26 March 2015 Available online --- Abstract Objective: Pelvic exenteration requires complete resection of the tumor with negative margins to be considered a curative surgery. The pur- pose of this review is to assess the optimal preoperative evaluation and surgical approach in patients with recurrent cervical cancer to in- crease the chances of achieving a curative surgery with decreased morbidity and mortality in the era of concurrent chemoradiotherapy. Methods: Review of English publications pertaining to cervical cancer within the last 25 years were included using PubMed and Cochrane Library searches. Results: Modern imaging (MRI and PET-CT) does not accurately identify local extension of microscopic disease and is inadequate for pre- operative planning of extent of resection. -
Management of Afferent Loop Obstruction: Reoperation Or Endoscopic and Percutaneous Interventions
See discussions, stats, and author profiles for this publication at: https://www.researchgate.net/publication/282163026 Management of afferent loop obstruction: Reoperation or endoscopic and percutaneous interventions Article in World Journal of Gastrointestinal Surgery · September 2015 DOI: 10.4240/wjgs.v7.i9.190 CITATIONS READS 32 427 2 authors, including: Konstantinos Tsalis Aristotle University of Thessaloniki 115 PUBLICATIONS 976 CITATIONS SEE PROFILE Some of the authors of this publication are also working on these related projects: Laparoscopic liver resection using ICG green visualization of hepatic structures View project Laparoscopic liver resection using ICG green visualization of hepatic structures View project All content following this page was uploaded by Konstantinos Tsalis on 26 May 2017. The user has requested enhancement of the downloaded file. Submit a Manuscript: http://www.wjgnet.com/esps/ World J Gastrointest Surg 2015 September 27; 7(9): 190-195 Help Desk: http://www.wjgnet.com/esps/helpdesk.aspx ISSN 1948-9366 (online) DOI: 10.4240/wjgs.v7.i9.190 © 2015 Baishideng Publishing Group Inc. All rights reserved. MINIREVIEWS Management of afferent loop obstruction: Reoperation or endoscopic and percutaneous interventions? Konstantinos Blouhos, Konstantinos Andreas Boulas, Konstantinos Tsalis, Anestis Hatzigeorgiadis Konstantinos Blouhos, Konstantinos Andreas Boulas, Anestis Abstract Hatzigeorgiadis, Department of General Surgery, General Hospital of Drama, 66100 Drama, Greece Afferent loop obstruction is a purely mechanical comp- lication that infrequently occurs following construction Konstantinos Tsalis, D’ Surgical Department, “G. Papanikolaou” of a gastrojejunostomy. The operations most commonly Hospital, Medical School, Aristotle University of Thessaloniki, associated with this complication are gastrectomy 54645 Thessaloniki, Greece with Billroth Ⅱ or Roux-en-Y reconstruction, and pancreaticoduodenectomy with conventional loop or Author contributions: Blouhos K designed the research; Boulas Roux-en-Y reconstruction. -

Pelvic Exenteration for the Management of Pelvic Malignancies
Chapter 7 Pelvic Exenteration for the Management of Pelvic Malignancies Daniel Paramythiotis, Konstantinia Kofina and Antonios Michalopoulos Additional information is available at the end of the chapter http://dx.doi.org/10.5772/61083 Abstract Pelvic exenteration is a surgical procedure first described by Brunschwig in 1948 as a curative or palliative treatment for pelvic and perineal tumors. It is actually a radical operation, involving en bloc resection of pelvic organs, including reproductive structures, bladder, and rectosigmoid. In patients with recurrent cervical and vaginal malignancy, it is associated with a 5-year survival of more than 50%. In spite of advances in surgical management, consequences such as stomas, are still frequently unavoidable for radical tumor excision. Most candidates for this procedure have been diagnosed with recurrent cervical cancer that has previously been treated with surgery and radiation, or radiation alone. Complications of pelvic exenteration are more severe than those of standard resection of a colorectal carcinoma, so it is not commonly performed, including wound infection, wound dehiscence (also described as burst abdomen) the creation of fistulae (perineal-fecal, uretero-vaginal, between conduit and perineal wound), urinary tract infections, perineal hernias and intestinal obstruction. Patients need to be carefully selected and counseled about risks and long-term issues related to the surgery. A comprehensive evaluation is required in order to exclude unresectable or metastatic disease. Evolution of the technique through laparoscopy and minimally invasive surgery may result in a reduction of morbidity and mortality. Keywords: Pelvic exenteration, gynecologic cancer 1. Introduction Pelvic exenteration was first described by Brunschwig and his colleagues of New York’s Memorial Hospital in 1948 [1] and was initially performed as a palliative surgical intervention © 2015 The Author(s). -

Treating Cervical Cancer If You've Been Diagnosed with Cervical Cancer, Your Cancer Care Team Will Talk with You About Treatment Options
cancer.org | 1.800.227.2345 Treating Cervical Cancer If you've been diagnosed with cervical cancer, your cancer care team will talk with you about treatment options. In choosing your treatment plan, you and your cancer care team will also take into account your age, your overall health, and your personal preferences. How is cervical cancer treated? Common types of treatments for cervical cancer include: ● Surgery for Cervical Cancer ● Radiation Therapy for Cervical Cancer ● Chemotherapy for Cervical Cancer ● Targeted Therapy for Cervical Cancer ● Immunotherapy for Cervical Cancer Common treatment approaches Depending on the type and stage of your cancer, you may need more than one type of treatment. For the earliest stages of cervical cancer, either surgery or radiation combined with chemo may be used. For later stages, radiation combined with chemo is usually the main treatment. Chemo (by itself) is often used to treat advanced cervical cancer. ● Treatment Options for Cervical Cancer, by Stage Who treats cervical cancer? Doctors on your cancer treatment team may include: 1 ____________________________________________________________________________________American Cancer Society cancer.org | 1.800.227.2345 ● A gynecologist: a doctor who treats diseases of the female reproductive system ● A gynecologic oncologist: a doctor who specializes in cancers of the female reproductive system who can perform surgery and prescribe chemotherapy and other medicines ● A radiation oncologist: a doctor who uses radiation to treat cancer ● A medical oncologist: a doctor who uses chemotherapy and other medicines to treat cancer Many other specialists may be involved in your care as well, including nurse practitioners, nurses, psychologists, social workers, rehabilitation specialists, and other health professionals. -
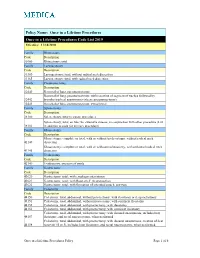
Once in a Lifetime Procedures Code List 2019 Effective: 11/14/2010
Policy Name: Once in a Lifetime Procedures Once in a Lifetime Procedures Code List 2019 Effective: 11/14/2010 Family Rhinectomy Code Description 30160 Rhinectomy; total Family Laryngectomy Code Description 31360 Laryngectomy; total, without radical neck dissection 31365 Laryngectomy; total, with radical neck dissection Family Pneumonectomy Code Description 32440 Removal of lung, pneumonectomy; Removal of lung, pneumonectomy; with resection of segment of trachea followed by 32442 broncho-tracheal anastomosis (sleeve pneumonectomy) 32445 Removal of lung, pneumonectomy; extrapleural Family Splenectomy Code Description 38100 Splenectomy; total (separate procedure) Splenectomy; total, en bloc for extensive disease, in conjunction with other procedure (List 38102 in addition to code for primary procedure) Family Glossectomy Code Description Glossectomy; complete or total, with or without tracheostomy, without radical neck 41140 dissection Glossectomy; complete or total, with or without tracheostomy, with unilateral radical neck 41145 dissection Family Uvulectomy Code Description 42140 Uvulectomy, excision of uvula Family Gastrectomy Code Description 43620 Gastrectomy, total; with esophagoenterostomy 43621 Gastrectomy, total; with Roux-en-Y reconstruction 43622 Gastrectomy, total; with formation of intestinal pouch, any type Family Colectomy Code Description 44150 Colectomy, total, abdominal, without proctectomy; with ileostomy or ileoproctostomy 44151 Colectomy, total, abdominal, without proctectomy; with continent ileostomy 44155 Colectomy, -

Pelvic Exenteration As Ultimate Ratio for Gynecologic Cancers: Single‑Center Analyses of 37 Cases
Archives of Gynecology and Obstetrics (2019) 300:161–168 https://doi.org/10.1007/s00404-019-05154-4 GYNECOLOGIC ONCOLOGY Pelvic exenteration as ultimate ratio for gynecologic cancers: single‑center analyses of 37 cases N. de Gregorio1 · A. de Gregorio1 · F. Ebner2 · T. W. P. Friedl1 · J. Huober1 · R. Hefty3 · M. Wittau4 · W. Janni1 · P. Widschwendter1 Received: 5 February 2019 / Accepted: 5 April 2019 / Published online: 22 April 2019 © Springer-Verlag GmbH Germany, part of Springer Nature 2019 Abstract Background Pelvic exenterations are a last resort procedure for advanced gynecologic malignancies with elevated risks in terms of patients’ morbidity. Methods This single-center analysis reports surgical details, outcome and survival of all patients treated with exenteration for non-ovarian gynecologic malignancies at our university hospital during a 13-year time period. We collected data regarding patients and tumor characteristics, surgical procedures, peri- and postoperative management, transfusions, complications, and analyzed the impact on survival outcomes. Results We identifed 37 patients between 2005 and 2013 with primary or relapsed cervical cancer (59.5%), vulvar cancer (24.3%) or endometrial cancer (16.2%). Median age was 60 years and most patients (73%) had squamous cell carcinomas. Median progression-free survival was 26.2 months and median overall survival was 49.9 months. The 5-year survival rates were 34.4% for progression-free survival and 46.4% for overall survival. There were no signifcant diferences in progres- sion-free survival and overall survival with regard to disease entity. Patients with tumor at the resection margins (R1) had a nearly signifcantly worse progression-free survival (median: 28.5 vs. -

Primary Pelvic Exenteration: Our Experience with 23 Patients from a Single Institution
EXPERIMENTAL AND THERAPEUTIC MEDICINE 22: 1060, 2021 Primary pelvic exenteration: Our experience with 23 patients from a single institution MIHAI GHEORGHE1, ALEXANDRA LAVINIA COZLEA1, SZILARD LEO KISS1, MIHAI STANCA1, MIHAI EMIL CĂPÎLNA1, NICOLAE BACALBAȘA2 and ANDREEA ANAMARIA MOLDOVAN3 1First Obstetrics and Gynecology Clinic, ‘George Emil Palade’ University of Medicine, Pharmacy, Science and Technology, 540136 Târgu Mureș; 2Department of Obstetrics and Gynecology, ‘Carol Davila’ University of Medicine and Pharmacy, 020022 Bucharest; 3Department of Infectious Diseases, Brașov County Emergency Hospital, 500326 Brașov, Romania Received May 24, 2021; Accepted June 23, 2021 DOI: 10.3892/etm.2021.10494 Abstract. This study was designed with an aim to share our Introduction experience of primary pelvic exenterations. The study included 23 patients with different types of pelvic cancer enrolled at a In patients with advanced primary or recurrent gyneco‑ single institution between November 2011 and July 2020. The logic (1), urologic or rectal cancers without metastatic disease, patient mean age was 55 years (range, 43‑72 years) and the extensive aggressive surgery such as pelvic exenteration may oncological indications included: Stage IVa cervical cancer be necessary for curative intent treatment (2). (11 cases, 48.9%), stage IVa endometrial cancer (1 case, 4.3%), Brunschwig was the first to describe pelvic exentera‑ stage IVa vaginal cancer (6 cases, 26%), stage IIIb bladder tion in the 1940s (3). Exenteration was initially considered cancer (3 cases, 13%), stage IIIc rectal cancer (1 case, 4.3%) as a palliative procedure for patients with extensive pelvic and undifferentiated pelvic sarcoma (1 case, 4.3%). Total, cancers, with an extremely high perioperative mortality rate anterior, and posterior pelvic exenterations were performed of 23%. -

Safe, Simple & Efficient Totally Laparoscopic Billroth II Gastrectomy
China Gastric Cancer Research Highlight Safe, simple & efficient totally laparoscopic Billroth II gastrectomy by only stapling devices Kirubakaran Malapan1, Chih-Kun Huang1,2 1Department of Bariatric and Metabolic International Surgery Centre, E-Da Hospital, Kaohsiung City, 82445, Taiwan; 2The First Affiliated Hospital of Guangzhou Medical University, Guangzhou 510120, China Corresponding to: Chih-Kun Huang, M.D., Director. Department of Bariatric and Metabolic International Surgery Centre, E-da Hospital, No 1, E-Da Rd., Yan-chau District, Kaohsiung City, Taiwan, 82445. Email: [email protected]. Submitted May 08, 2013. Accepted for publication May 28, 2013. doi: 10.3978/j.issn.2224-4778.2013.05.28 Scan to your mobile device or view this article at: http://www.amepc.org/tgc/article/view/2081/2870 Gastric cancer is the fourth most common cancer diagnosis combination of both. Du J et al. reported their experience worldwide in men with an expected incidence of 640,000 of intracorporeal gastrojejunal anastomosis using a two cases and the fifth most common in women with an expected layer hand-sewn technique (9), whereas Ruiz et al. described incidence of 350,000 cases in 2011 (1). Approximately, 8% a 4-layer closure using continuous absorbable sutures (10). of total cases and 10% of annual cancer deaths worldwide Hand-sewn anastomosis requires advanced laparoscopic are attributed to this dreaded disease. Surgical resection skills and is considered to be time-consuming, but has offers the only durable cure from gastric cancer (2). Since the advantage of avoiding the risk of wound infection and the introduction of Billroth’s procedure of gastrectomy and hernias, which occur as a result of manipulation by a circular reconstruction in 1881, surgical techniques in gastric surgery stapler. -

Electrohydraulic Lithotripsy of an Impacted Enterolith Causing Acute Afferent Loop Syndrome
CASE REPORT Print ISSN 2234-2400 / On-line ISSN 2234-2443 Clin Endosc 2014;47:367-370 http://dx.doi.org/10.5946/ce.2014.47.4.367 Open Access Electrohydraulic Lithotripsy of an Impacted Enterolith Causing Acute Afferent Loop Syndrome Young Sin Cho, Tae Hoon Lee, Soon Oh Hwang, Sunhyo Lee, Yunho Jung, Il-Kwun Chung, Sang-Heum Park and Sun-Joo Kim Division of Gastroenterology, Department of Internal Medicine, Soonchunhyang University Cheonan Hospital, Soonchunhyang University College of Medicine, Cheonan, Korea Afferent loop syndrome caused by an impacted enterolith is very rare, and endoscopic removal of the enterolith may be difficult if a stricture is present or the normal anatomy has been altered. Electrohydraulic lithotripsy is commonly used for endoscopic fragmenta- tion of biliary and pancreatic duct stones. A 64-year-old man who had undergone subtotal gastrectomy and gastrojejunostomy presented with acute, severe abdominal pain for a duration of 2 hours. Initially, he was diagnosed with acute pancreatitis because of an elevated amy- lase level and pain, but was finally diagnosed with acute afferent loop syndrome when an impacted enterolith was identified by comput- ed tomography. We successfully removed the enterolith using direct electrohydraulic lithotripsy conducted using a transparent cap-fitted endoscope without complications. We found that this procedure was therapeutically beneficial. Key Words: Afferent loop syndrome; Enterolith; Electrohydraulic lithotripsy INTRODUCTION cutaneous enterostomy has been considered as a palliative method. However, maturation of the percutaneous tract can Afferent loop syndrome (ALS) is characterized by the ac- occur very slowly prior to the procedure, and may cause dis- cumulation of bile acid and pancreatic juice, and distention comfort and pain. -

Introduction to Pelvic Obstetric and Gynaecology Physiotherapy
INTRODUCTION TO PELVIC OBSTETRIC AND GYNAECOLOGY PHYSIOTHERAPY An Educational Resource Published by the Pelvic, Obstetric and Gynaecology Physiotherapy Contact: POGP Administration, Fitwise Management Ltd., Drumcross Hall, Bathgate, West Lothian EH48 4JT T: 01506 811077 E: [email protected] or visit the POGP website www.pogp.org.uk for further information. ©POGP 2015 for review 2018 Contents CCCCC Introduction .........................................................................................................3 Core skill framework.............................................................................................4 Obstetrics..............................................................................................................5 Urology, Gynaecology and Colorectal..................................................................8 Breast Oncology……………………………………………………..………...………………..….……..17 Appendices 1. Obstetric abbreviations ..........................................................................19 2. Obstetric terminology ............................................................................20 3. Urology/ Gynaecology terminology……………………………………..……………..26 4. Gynaecology surgery terminology…………………………………………..………....29 5. Colorectal terminology…………………………………………………..………………..…31 6. Advanced clinical objectives for performing internal examinations…….34 C o n t e n t s 1 T H R E Introduction It is acknowledged that physiotherapists may become involved in the management of pelvic, obstetric and gynaecology patients -

Coders' Desk Reference for ICD-10-PCS Procedures
2 0 2 DESK REFERENCE 1 ICD-10-PCS Procedures ICD-10-PCS for DeskCoders’ Reference Coders’ Desk Reference for ICD-10-PCS Procedures Clinical descriptions with answers to your toughest ICD-10-PCS coding questions Sample 2021 optum360coding.com Contents Illustrations ..................................................................................................................................... xi Introduction .....................................................................................................................................1 ICD-10-PCS Overview ...........................................................................................................................................................1 How to Use Coders’ Desk Reference for ICD-10-PCS Procedures ...................................................................................2 Format ......................................................................................................................................................................................3 ICD-10-PCS Official Guidelines for Coding and Reporting 2020 .........................................................7 Conventions ...........................................................................................................................................................................7 Medical and Surgical Section Guidelines (section 0) ....................................................................................................8 Obstetric Section Guidelines (section -

OBGYN Outpatient Surgery Coding
OBGYN Outpatient Surgery Coding Anatomy Anatomy • Hyster/o – uterus, womb • Uter/o – uterus, womb • Metr/o – uterus, womb • Salping/o – tube, usually fallopian tube • Oophor/o – ovary • Ovari/o - ovary Terminology • Colpo – vagina • Cervic/o – cervix, lower part of the uterus, the “neck” • Episi/o – vulva • Vulv/o – vulva • Perine/o – the space between the anus and vulva Hysterectomy • A hysterectomy is an operation to remove a woman's uterus. • A woman may have a hysterectomy for different reasons, including: • Uterine fibroids that cause pain • bleeding, or other problems. • Uterine prolapse, which is a sliding of the uterus from its normal position into the vaginal canal. Hysterectomy • There are around 30 hysterectomy CPT codes. • To find the correct code you have to first check: • the surgical approach and • extent of the procedure. Surgical Approaches • Abdominal – the uterus is removed via an incision in the lower abdomen • Vaginal – the uterus is removed via an incision in the vagina • Laparoscopic – the procedure is performed using a laparoscope , inserted via several small incisions in the body. • Their are also CPT codes for laparoscopic-assisted vaginal approach. In this procedure ,the scope is inserted via a small incisions in the vagina. Extent of Procedure • Total hysterectomy: It includes laparoscopically detaching the entire uterine cervix and body from the surrounding supporting structures and suturing the vaginal cuff. It includes bivalving, coring, or morcellating the excised tissues, as required. The uterus is then removed through the vagina or abdomen. • Subtotal, partial or supracervical hysterectomy: It is the removal of the fundus or op portion of the uterus only, leaving the cervix in place.