Why Chloroplasts and Mitochondria Retain Their Own Genomes
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Reconstructing Mitochondrial Evolution?? Morphological Diversity Mitochondrial Diversity???
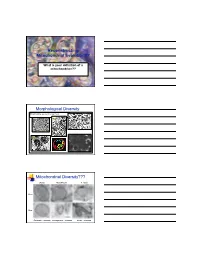
Reconstructing Mitochondrial Evolution?? What is your definition of a mitochondrion?? Morphological Diversity Mitochondria as we all know them: Suprarenal gland Liver cell Plasma cell Adrenal cortex Mitochondrial Diversity??? Chicken Neocallimastix T. foetus 100 nm 50 nm Entamoeba - mitosome microsporidian - mitosome Giardia - mitosome Reconstructing Evolution Mitochondrial evolution well established endosymbiotic theory α-proteobacterium - Rickettsia prowazekii Hydrogenosomal evolution No DNA NOW 2 examples Nyctotherus and Blastocystis (MLO) Several proteins similar to mitochondria Mitosome evolution No DNA Few proteins identified similar to mitochondria Origins via Endosymbiosis Current dogma - mitochondria and related Aerobic α-proteobacterium prokaryote gave rise to present day mitochondria. organelles arose just once in evolution Are hydrogenosomes and mitosomes of anaerobic protists derived from the same proto-mitochondrion? Evidence for: accumulating evidence for several proteins that are currently found in mitochondria - Proteins of Fe-S cluster formation. Scenario A Common ancestor facultative Scenario B organism? degenerate mitochondrion invoke lateral gene transfer from anaerobic prokaryotes Hypotheses for Mito Acquisition Mitochondrion-related Organelles 2 way to interpret this summary - can you think of both? Groups with mitochondrial homologues Hjort et al Phil. Trans. R. Soc. B 2010 365, 713-727 Origin of Hydrogenosomes (Mitosomes?) 1. a)Conversion of Mitochondria HYD b)Common ancestor with mitochondria ? HYD 2. Independent Origin α-proteobacterium ? - anaerobic bacterium HYD Organelles - origins and biogenesis Approaches: (1) Conduct phylogenetic analyses of similar proteins Hsp70 Fd Hsp60 Isc subunits (2) Examine protein targeting to the organelle matrix protein targeting membrane protein targeting (3) Characterize membrane/translocation components These components could have evolved as the endosymbiont was converted to organelle. Reveals evolutionary history. -

Virus World As an Evolutionary Network of Viruses and Capsidless Selfish Elements
Virus World as an Evolutionary Network of Viruses and Capsidless Selfish Elements Koonin, E. V., & Dolja, V. V. (2014). Virus World as an Evolutionary Network of Viruses and Capsidless Selfish Elements. Microbiology and Molecular Biology Reviews, 78(2), 278-303. doi:10.1128/MMBR.00049-13 10.1128/MMBR.00049-13 American Society for Microbiology Version of Record http://cdss.library.oregonstate.edu/sa-termsofuse Virus World as an Evolutionary Network of Viruses and Capsidless Selfish Elements Eugene V. Koonin,a Valerian V. Doljab National Center for Biotechnology Information, National Library of Medicine, Bethesda, Maryland, USAa; Department of Botany and Plant Pathology and Center for Genome Research and Biocomputing, Oregon State University, Corvallis, Oregon, USAb Downloaded from SUMMARY ..................................................................................................................................................278 INTRODUCTION ............................................................................................................................................278 PREVALENCE OF REPLICATION SYSTEM COMPONENTS COMPARED TO CAPSID PROTEINS AMONG VIRUS HALLMARK GENES.......................279 CLASSIFICATION OF VIRUSES BY REPLICATION-EXPRESSION STRATEGY: TYPICAL VIRUSES AND CAPSIDLESS FORMS ................................279 EVOLUTIONARY RELATIONSHIPS BETWEEN VIRUSES AND CAPSIDLESS VIRUS-LIKE GENETIC ELEMENTS ..............................................280 Capsidless Derivatives of Positive-Strand RNA Viruses....................................................................................................280 -

Interdependence Between Chloroplasts and Mitochondria in the Light and the Dark
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Elsevier - Publisher Connector Biochimica et Biophysica Acta 1366 (1998) 235^255 Review Interdependence between chloroplasts and mitochondria in the light and the dark Marcel H.N. Hoefnagel a, Owen K. Atkin b, Joseph T. Wiskich a;* a Department of Botany, University of Adelaide, Adelaide, SA 5005, Australia b Research School for Biological Sciences, Australian National University, Canberra, ACT 0200, Australia Received 21 April 1998; revised 3 June 1998; accepted 10 June 1998 ß 1998 Elsevier Science B.V. All rights reserved. Keywords: Chloroplast; Chlororespiration; Excess reductant; Metabolite exchange; Mitochondrion; Photosynthesis; Respiration Contents 1. Introduction .......................................................... 236 2. Interactions between organelles depends on metabolite exchange . .................. 236 2.1. ATP exchange ..................................................... 236 2.2. Transport of reducing equivalents across membranes . ....................... 237 2.3. Exchange of carbon compounds ....................................... 238 3. Respiration in the light . ................................................. 239 3.1. Does respiration continue in the light? . .................................. 239 3.2. Substrates for the mitochondria in the light . ............................ 240 3.3. ATP supply in the light: chloroplasts versus mitochondria . .................. 240 3.4. Adenylate control of respiration in the light . -

Bioenergetic Abnormalities in Schizophrenia
Bioenergetic abnormalities in schizophrenia A dissertation submitted to the Graduate School of the University of Cincinnati in partial fulfillment of the requirements for the degree of Doctor of Philosophy in the Graduate Program in Neuroscience of the College of Medicine by Courtney René Sullivan B.S. University of Pittsburgh, 2013 Dissertation Committee: Mark Baccei, Ph.D. (chair) Robert McCullumsmith, M.D., Ph.D. (advisor) Michael Lieberman, Ph.D. Temugin Berta, Ph.D. Robert McNamara, Ph.D. ABSTRACT Schizophrenia is a devastating illness that affects over 2 million people in the U.S. and displays a wide range of psychotic symptoms, as well as cognitive deficits and profound negative symptoms that are often treatment resistant. Cognition is intimately related to synaptic function, which relies on the ability of cells to obtain adequate amounts of energy. Studies have shown that disrupting bioenergetic pathways affects working memory and other cognitive behaviors. Thus, investigating bioenergetic function in schizophrenia could provide important insights into treatments or prevention of cognitive disorders. There is accumulating evidence of bioenergetic dysfunction in chronic schizophrenia, including deficits in energy storage and usage in the brain. However, it is unknown if glycolytic pathways are disrupted in this illness. This dissertation employs a novel reverse translational approach to explore glycolytic pathways in schizophrenia, effectively combining human postmortem studies with bioinformatic analyses to identify possible treatment strategies, which we then examine in an animal model. To begin, we characterized a major pathway supplying energy to neurons (the lactate shuttle) in the dorsolateral prefrontal cortex (DLPFC) in chronic schizophrenia. We found a significant decrease in the activity of two key glycolytic enzymes in schizophrenia (hexokinase, HXK and phosphofructokinase, PFK), suggesting a decrease in the capacity to generate bioenergetic intermediates through glycolysis in this illness. -

Evidence Supporting an Antimicrobial Origin of Targeting Peptides to Endosymbiotic Organelles
cells Article Evidence Supporting an Antimicrobial Origin of Targeting Peptides to Endosymbiotic Organelles Clotilde Garrido y, Oliver D. Caspari y , Yves Choquet , Francis-André Wollman and Ingrid Lafontaine * UMR7141, Institut de Biologie Physico-Chimique (CNRS/Sorbonne Université), 13 Rue Pierre et Marie Curie, 75005 Paris, France; [email protected] (C.G.); [email protected] (O.D.C.); [email protected] (Y.C.); [email protected] (F.-A.W.) * Correspondence: [email protected] These authors contributed equally to this work. y Received: 19 June 2020; Accepted: 24 July 2020; Published: 28 July 2020 Abstract: Mitochondria and chloroplasts emerged from primary endosymbiosis. Most proteins of the endosymbiont were subsequently expressed in the nucleo-cytosol of the host and organelle-targeted via the acquisition of N-terminal presequences, whose evolutionary origin remains enigmatic. Using a quantitative assessment of their physico-chemical properties, we show that organelle targeting peptides, which are distinct from signal peptides targeting other subcellular compartments, group with a subset of antimicrobial peptides. We demonstrate that extant antimicrobial peptides target a fluorescent reporter to either the mitochondria or the chloroplast in the green alga Chlamydomonas reinhardtii and, conversely, that extant targeting peptides still display antimicrobial activity. Thus, we provide strong computational and functional evidence for an evolutionary link between organelle-targeting and antimicrobial peptides. Our results support the view that resistance of bacterial progenitors of organelles to the attack of host antimicrobial peptides has been instrumental in eukaryogenesis and in the emergence of photosynthetic eukaryotes. Keywords: Chlamydomonas; targeting peptides; antimicrobial peptides; primary endosymbiosis; import into organelles; chloroplast; mitochondrion 1. -

Mitosomes in Entamoeba Histolytica Contain a Sulfate Activation Pathway
Mitosomes in Entamoeba histolytica contain a sulfate activation pathway Fumika Mi-ichi, Mohammad Abu Yousuf, Kumiko Nakada-Tsukui, and Tomoyoshi Nozaki1 Department of Parasitology, National Institute of Infectious Diseases, Tokyo 162-8640, Japan Edited by Andrew Roger, Dalhousie University, Halifax, NS, Canada, and accepted by the Editorial Board October 19, 2009 (received for review June 25, 2009) Hydrogenosomes and mitosomes are mitochondrion-related or- and M. balamuthi lack the ISC system, and instead possess the ganelles in anaerobic/microaerophilic eukaryotes with highly re- nitrogen fixation (NIF) system, which is most likely derived from an duced and divergent functions. The full diversity of their content ancestral nitrogen fixing -proteobacterium by lateral gene transfer and function, however, has not been fully determined. To under- (22, 24). Only 5 proteins have been demonstrated in E. histolytica stand the central role of mitosomes in Entamoeba histolytica,a mitosomes: Cpn60 (8–10, 12), Cpn10 (13), mitochondrial Hsp70 parasitic protozoon that causes amoebic dysentery and liver ab- (11, 15), pyridine nucleotide transhydrogenase (PNT) (2, 8), and scesses, we examined the proteomic profile of purified mitosomes. mitochondria carrier family (MCF, ADP/ATP transporter) (14), Using 2 discontinuous Percoll gradient centrifugation and MS and the central role of mitosomes in E. histolytica remains unknown. analysis, we identified 95 putative mitosomal proteins. Immuno- Analysis of the genome of E. histolytica has not revealed any fluorescence assay showed that 3 proteins involved in sulfate additional information regarding the function of mitosomes and activation, ATP sulfurylase, APS kinase, and inorganic pyrophos- thus, a proteomic analysis of mitosomes seems to be the best phatase, as well as sodium/sulfate symporter, involved in sulfate approach to understand its structure and function (1, 2). -

A Novel ADP/ATP Transporter in the Mitosome of the Microaerophilic Human Parasite Entamoeba Histolytica
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Elsevier - Publisher Connector Current Biology, Vol. 15, 737–742, April 26, 2005, ©2005 Elsevier Ltd All rights reserved. DOI 10.1016/j.cub.2005.02.068 A Novel ADP/ATP Transporter in the Mitosome of the Microaerophilic Human Parasite Entamoeba histolytica Ka Wai Chan,1,5 Dirk-Jan Slotboom,1,5 Sian Cox,3 ses confirm that the Entamoeba ADP/ATP carrier is T. Martin Embley,2,* Olivier Fabre,1 distinct from archetypal mitochondrial ADP/ATP carri- Mark van der Giezen,3,6 Marilyn Harding,1 ers, an observation that is supported by its different David S. Horner,4 Edmund R.S. Kunji,1,* substrate and inhibitor specificity. Because many Gloria León-Avila,3,7 and Jorge Tovar3 functions of yeast and human mitochondria rely on 1Dunn Human Nutrition Unit solutes transported by specialized members of this Medical Research Council family, the Entamoeba mitosome must contain only Hills Road a small subset of these processes requiring adenine Cambridge CB2 2XY nucleotide exchange. United Kingdom 2 School of Biology The Devonshire Building Results and Discussion University of Newcastle upon Tyne Newcastle NE1 7RU Identification of an MCF Homolog United Kingdom on the Entamoeba histolytica Genome 3 School of Biological Sciences We identified a homolog of the mitochondrial carrier Royal Holloway family (MCF) on the Entamoeba histolytica genome (TIGR) University of London with BLAST searches. The translated protein contains a Egham, Surrey TW20 0EX tripartite structure [8], signature motifs (IPR001993), and United Kingdom other features typical of MCF members (Figure 1A). -

Biochemical Fossils of the Ancient Transition from Geoenergetics to Bioenergetics in Prokaryotic One Carbon Compound Metabolism☆
BBABIO-47248; No. of pages: 18; 4C: 4, 6, 7, 9 Biochimica et Biophysica Acta xxx (2014) xxx–xxx Contents lists available at ScienceDirect Biochimica et Biophysica Acta journal homepage: www.elsevier.com/locate/bbabio Biochemical fossils of the ancient transition from geoenergetics to bioenergetics in prokaryotic one carbon compound metabolism☆ Filipa L. Sousa, William F. Martin ⁎ Institute for Molecular Evolution,University of Düsseldorf, 40225 Düsseldorf, Germany article info abstract Article history: The deep dichotomy of archaea and bacteria is evident in many basic traits including ribosomal protein compo- Received 26 October 2013 sition, membrane lipid synthesis, cell wall constituents, and flagellar composition. Here we explore that deep di- Received in revised form 31 January 2014 chotomy further by examining the distribution of genes for the synthesis of the central carriers of one carbon Accepted 3 February 2014 units, tetrahydrofolate (H F) and tetrahydromethanopterin (H MPT), in bacteria and archaea. The enzymes un- Available online xxxx 4 4 derlying those distinct biosynthetic routes are broadly unrelated across the bacterial–archaeal divide, indicating Keywords: that the corresponding pathways arose independently. That deep divergence in one carbon metabolism is mir- Pterins rored in the structurally unrelated enzymes and different organic cofactors that methanogens (archaea) and Hydrothermal vents acetogens (bacteria) use to perform methyl synthesis in their H4F- and H4MPT-dependent versions, respectively, Origin of life of the acetyl-CoA pathway. By contrast, acetyl synthesis in the acetyl-CoA pathway — from a methyl group, CO2 Methanogens and reduced ferredoxin — is simpler, uniform and conserved across acetogens and methanogens, and involves Acetogens only transition metals as catalysts. -

Calvin Cycle E
Photosynthesis Photosynthesis is the process by which plants use sunlight (light energy) to produce glucose from carbon dioxide and water, with oxygen as a byproduct. This process occurs LIGHT STROMA in the chloroplasts in a plant cell and has DEPENDENT Cytochrome b f Reduced REACTIONS two stages – the light-dependent reactions 6 NADP NADP + 1. Light activation of and the light-independent reactions. H ATP synthase photocentres It all starts with the sunlight hitting e- PSII 2. Photolysis of water - + the fi rst photocentre (PSII). (P68 e PSI H e- (P 3. Electron transport e- ATP e- + 4. Pumping H into the + O H thylakoid space ADP + Pi H O + + H 5. Synthesis of ATP H 6. Reduction of NADP Photolysis THYLAKOID SPACE CO OXYGEN Thylakoid membrane GLUCOSE REDUCED NADP & ATP NADP REDUCED Glucose is used in respiration for energy. Glucose is converted to: 1. Cellulose for cell walls 2. Sucrose for transport 3. Starch for storage ADP + Pi & NADP + Pi ADP CHLOROPLAST CALVIN C LIGHT CO YCL E ( Six INDEPENDENT RuBisCO tu rn enzyme BON FIXAT s CAR ION to REACTIONS p ro d u c Calvin cycle e RuBP GP g l 1. Carbon fi xation u Ribulose bisphosphate Glycerate phosphate c o s e ) 2. Reduction P B 3. Regeneration of RuBP u R ATP F ADP + Pi O N ADP + Pi O R I ATP E T D A R Reduced NADP U E C N T E I O G NADP N E R GALP Glyceraldehyde phosphate Hexose Glucose LAMELLA THYLAKOIDS STROMA KEY TO SYMBOLS INNER CHLOROPLAST MEMBRANE Carbon atom: Electron transport: e- OUTER CHLOROPLAST MEMBRANE H+ movement: GLOSSARY ATP synthase: An enzyme that catalyses the Ferrodoxin: An electron carrier sitting just Light-dependent reactions: The fi rst stage Photosystem I (PSI): The second photosystem Plastoquinone: A molecule that is reduced Regeneration of RuBP: The third stage of Stroma: The aqueous solution that fi lls the synthesis of ATP from ADP and inorganic outside the thylakoid in the chloroplast of photosynthesis. -

Bisphosphate Carboxylase/Oxygenase Activation in Tomato (Lycopersicon Esculentum Mil!.)
Plant Physiol. (1995) 107: 585-591 The Effects of Chilling in the Light on Ribulose-1,5- Bisphosphate Carboxylase/Oxygenase Activation in Tomato (Lycopersicon esculentum Mil!.) George T. Byrd’, Donald R. Ort, and William 1. Ogren* Photosynthesis Research Unit, Agricultura1 Research Service, United States Department of Agriculture (G.T.B., D.R.O., W.L.O.), and Department of Plant Biology, University of lllinois at Urbana-Champaign (D.R.O., W.L.O.), Urbana, lllinois 61801 reduced leve1 of RuBP. The bisphosphatases are activated Photosynthesis rate, ribulose-l,5-bisphosphate carboxylase/oxy- by the Fd/thioredoxin system (Buchanan, 1980), which genase (Rubisco) activation state, and ribulose bisphosphate con- may be affected by the light-chilling regime. Thus, in to- centration were reduced after exposing tomato (Lycopersicon es- mato, chilling in the light appears to limit the capacity of culentum Mill.) plants to light at 4°C for 6 h. Analysis of lysed and leaves to regenerate RuBP, the substrate in photosynthetic reconstituted chloroplasts showed that activity of the thylakoid CO, fixation catalyzed by the enzyme Rubisco (EC membrane was inhibited and that Rubisco, Rubisco activase, and 4.1.1.39). other soluble factors were not affected. Leaf photosynthesis rates Rubisco activity also has been reported to be directly and the ability of chilled thylakoid membranes to promote Rubisco impaired during chilling in the light in short-term (Sas- activation recovered after 24 h at 25°C. Thylakoid membranes from control tomato plants were as effective as spinach thylakoids in senrath et al., 1990) and long-term (Briiggemann et al., activating spinach Rubisco in the presence of spinach Rubisco ac- 1992) experiments. -

The Photosynthetic Endosymbiont in Cryptomonad Cells Produces Both Chloroplast and Cytoplasmic-Type Ribosomes
Journal of Cell Science 107, 649-657 (1994) 649 Printed in Great Britain © The Company of Biologists Limited 1994 JCS6601 The photosynthetic endosymbiont in cryptomonad cells produces both chloroplast and cytoplasmic-type ribosomes Geoffrey I. McFadden1,*, Paul R. Gilson1 and Susan E. Douglas2 1Plant Cell Biology Research Centre, School of Botany, University of Melbourne, Parkville, Victoria, 3052, Australia 2Institute of Marine Biosciences, National Research Council of Canada, 1411 Oxford St, Halifax, Nova Scotia B3H 3Z1, Canada *Author for correspondence SUMMARY Cryptomonad algae contain a photosynthetic, eukaryotic tion machinery. We also localized transcripts of the host endosymbiont. The endosymbiont is much reduced but nucleus rRNA gene. These transcripts were found in the retains a small nucleus. DNA from this endosymbiont nucleolus of the host nucleus, and throughout the host nucleus encodes rRNAs, and it is presumed that these cytoplasm, but never in the endosymbiont compartment. rRNAs are incorporated into ribosomes. Surrounding the Our rRNA localizations indicate that the cryptomonad cell endosymbiont nucleus is a small volume of cytoplasm produces two different of sets of cytoplasmic-type proposed to be the vestigial cytoplasm of the endosymbiont. ribosomes in two separate subcellular compartments. The If this compartment is indeed the endosymbiont’s results suggest that there is no exchange of rRNAs between cytoplasm, it would be expected to contain ribosomes with these compartments. We also used the probe specific for the components encoded by the endosymbiont nucleus. In this endosymbiont rRNA gene to identify chromosomes from paper, we used in situ hybridization to localize rRNAs the endosymbiont nucleus in pulsed field gel electrophore- encoded by the endosymbiont nucleus of the cryptomonad sis. -

Molecular Biology of the Cell 6Th Edition
753 CHAPTER Energy Conversion: Mitochondria and Chloroplasts 14 To maintain their high degree of organization in a universe that is constantly drift- IN THIS CHAPTER ing toward chaos, cells have a constant need for a plentiful supply of ATP, as we have explained in Chapter 2. In eukaryotic cells, most of the ATP that powers life THE MITOCHONDRION processes is produced by specialized, membrane-enclosed, energy-converting organelles. Tese are of two types. Mitochondria, which occur in virtually all cells THE PROTON PUMPS OF THE of animals, plants, and fungi, burn food molecules to produce ATP by oxidative ELECTRON-TRANSPORT CHAIN phosphorylation. Chloroplasts, which occur only in plants and green algae, har- ness solar energy to produce ATP by photosynthesis. In electron micrographs, the ATP PRODUCTION IN most striking features of both mitochondria and chloroplasts are their extensive MITOCHONDRIA internal membrane systems. Tese internal membranes contain sets of mem- brane protein complexes that work together to produce most of the cell’s ATP. In CHLOROPLASTS AND bacteria, simpler versions of essentially the same protein complexes produce ATP, PHOTOSYNTHESIS but they are located in the cell’s plasma membrane (Figure 14–1). Comparisons of DNA sequences indicate that the energy-converting organ- THE GENETIC SYSTEMS elles in present-day eukaryotes originated from prokaryotic cells that were endo- OF MITOCHONDRIA AND cytosed during the evolution of eukaryotes (discussed in Chapter 1). This explains CHLOROPLASTS why mitochondria and chloroplasts contain their own DNA, which still encodes a subset of their proteins. Over time, these organelles have lost most of their own genomes and become heavily dependent on proteins that are encoded by genes in the nucleus, synthesized in the cytosol, and then imported into the organelle.