Why Chloroplasts and Mitochondria Retain Their Own Genomes and Genetic Systems
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The First Cells Were Most Likely Very Simple Prokaryotic Forms. Ra- Spirochetes
T HE O RIGIN OF E UKARYOTIC C ELLS The first cells were most likely very simple prokaryotic forms. Ra- spirochetes. Ingestion of prokaryotes that resembled present-day diometric dating indicates that the earth is 4 to 5 billion years old cyanobacteria could have led to the endosymbiotic development of and that prokaryotes may have arisen more than 3.5 billion years chloroplasts in plants. ago. Eukaryotes are thought to have first appeared about 1.5 billion Another hypothesis for the evolution of eukaryotic cells proposes years ago. that the prokaryotic cell membrane invaginated (folded inward) to en- The eukaryotic cell might have evolved when a large anaerobic close copies of its genetic material (figure 1b). This invagination re- (living without oxygen) amoeboid prokaryote ingested small aerobic (liv- sulted in the formation of several double-membrane-bound entities ing with oxygen) bacteria and stabilized them instead of digesting them. (organelles) in a single cell. These entities could then have evolved This idea is known as the endosymbiont hypothesis (figure 1a) and into the eukaryotic mitochondrion, nucleus, and chloroplasts. was first proposed by Lynn Margulis, a biologist at Boston Univer- Although the exact mechanism for the evolution of the eu- sity. (Symbiosis is an intimate association between two organisms karyotic cell will never be known with certainty, the emergence of of different species.) According to this hypothesis, the aerobic bac- the eukaryotic cell led to a dramatic increase in the complexity and teria developed into mitochondria, which are the sites of aerobic diversity of life-forms on the earth. At first, these newly formed eu- respiration and most energy conversion in eukaryotic cells. -

Bioenergetics and Metabolism Mitochondria Chloroplasts
Bioenergetics and metabolism Mitochondria Chloroplasts Peroxisomes B. Balen Chemiosmosis common pathway of mitochondria, chloroplasts and prokaryotes to harness energy for biological purposes → chemiosmotic coupling – ATP synthesis (chemi) + membrane transport (osmosis) Prokaryotes – plasma membrane → ATP production Eukaryotes – plasma membrane → transport processes – membranes of cell compartments – energy-converting organelles → production of ATP • Mitochondria – fungi, animals, plants • Plastids (chloroplasts) – plants The essential requirements for chemiosmosis source of high-energy e- membrane with embedded proton pump and ATP synthase energy from sunlight or the pump harnesses the energy of e- transfer to pump H+→ oxidation of foodstuffs is proton gradient across the membrane used to create H+ gradient + across a membrane H gradient serves as an energy store that can be used to drive ATP synthesis Figures 14-1; 14-2 Molecular Biology of the Cell (© Garland Science 2008) Electron transport processes (A) mitochondrion converts energy from chemical fuels (B) chloroplast converts energy from sunlight → electron-motive force generated by the 2 photosystems enables the chloroplast to drive electron transfer from H2O to carbohydrate → chloroplast electron transfer is opposite of electron transfer in a mitochondrion Figure 14-3 Molecular Biology of the Cell (© Garland Science 2008) Carbohydrate molecules and O2 are products of the chloroplast and inputs for the mitochondrion Figure 2-41; 2-76 Molecular Biology of the Cell (© Garland -

Genome-Wide Identification, Classification, and Analysis of Two-Component Signal System Genes in Maize
Genome-wide identification, classification, and analysis of two-component signal system genes in maize Z.X. Chu*, Q. Ma*, Y.X. Lin, X.L. Tang, Y.Q. Zhou, S.W. Zhu, J. Fan and B.J. Cheng Anhui Province Key Laboratory of Crop Biology, School of Life Science, Anhui Agricultural University, Hefei, China *These authors contributed equally to this study. Corresponding authors: B.J. Cheng / J. Fan E-mail: [email protected] / [email protected] Genet. Mol. Res. 10 (4): 3316-3330 (2011) Received March 23, 2011 Accepted August 31, 2011 Published December 8, 2011 DOI http://dx.doi.org/10.4238/2011.December.8.3 ABSTRACT. Cytokinins play many vital roles in plant development and physiology. In plants, cytokinin signals are sensed and transduced by the two-component signal system. This signaling cascade is typically com- posed of three proteins: a sensory histidine kinase, a histidine phosphotrans- fer protein, and a response regulator. Through a comprehensive genome- wide analysis of the maize (Zea mays) genome, 48 genes were identified, including 11 ZmHKs, 9 ZmHPs, and 28 ZmRRs (21 A-type ZmRRs and 7 B-type ZmRRs). Using maize genome sequence databases, we analyzed conserved protein motifs and established phylogenetic relationships based on gene structure, homology, and chromosomal location. The duplication of these two-component system genes in the maize genome corresponded to the clusters of these genes in the phylogenetic trees. Sequence analysis of the duplicate genes demonstrated that one gene may be in gene duplica- tion, and that there was significant variation in the evolutionary history of the different gene families. -
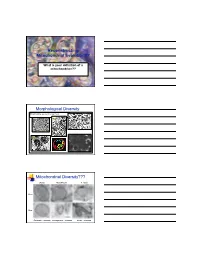
Reconstructing Mitochondrial Evolution?? Morphological Diversity Mitochondrial Diversity???
Reconstructing Mitochondrial Evolution?? What is your definition of a mitochondrion?? Morphological Diversity Mitochondria as we all know them: Suprarenal gland Liver cell Plasma cell Adrenal cortex Mitochondrial Diversity??? Chicken Neocallimastix T. foetus 100 nm 50 nm Entamoeba - mitosome microsporidian - mitosome Giardia - mitosome Reconstructing Evolution Mitochondrial evolution well established endosymbiotic theory α-proteobacterium - Rickettsia prowazekii Hydrogenosomal evolution No DNA NOW 2 examples Nyctotherus and Blastocystis (MLO) Several proteins similar to mitochondria Mitosome evolution No DNA Few proteins identified similar to mitochondria Origins via Endosymbiosis Current dogma - mitochondria and related Aerobic α-proteobacterium prokaryote gave rise to present day mitochondria. organelles arose just once in evolution Are hydrogenosomes and mitosomes of anaerobic protists derived from the same proto-mitochondrion? Evidence for: accumulating evidence for several proteins that are currently found in mitochondria - Proteins of Fe-S cluster formation. Scenario A Common ancestor facultative Scenario B organism? degenerate mitochondrion invoke lateral gene transfer from anaerobic prokaryotes Hypotheses for Mito Acquisition Mitochondrion-related Organelles 2 way to interpret this summary - can you think of both? Groups with mitochondrial homologues Hjort et al Phil. Trans. R. Soc. B 2010 365, 713-727 Origin of Hydrogenosomes (Mitosomes?) 1. a)Conversion of Mitochondria HYD b)Common ancestor with mitochondria ? HYD 2. Independent Origin α-proteobacterium ? - anaerobic bacterium HYD Organelles - origins and biogenesis Approaches: (1) Conduct phylogenetic analyses of similar proteins Hsp70 Fd Hsp60 Isc subunits (2) Examine protein targeting to the organelle matrix protein targeting membrane protein targeting (3) Characterize membrane/translocation components These components could have evolved as the endosymbiont was converted to organelle. Reveals evolutionary history. -

Origin and Evolution of Plastids and Mitochondria : the Phylogenetic Diversity of Algae
Cah. Biol. Mar. (2001) 42 : 11-24 Origin and evolution of plastids and mitochondria : the phylogenetic diversity of algae Catherine BOYEN*, Marie-Pierre OUDOT and Susan LOISEAUX-DE GOER UMR 1931 CNRS-Goëmar, Station Biologique CNRS-INSU-Université Paris 6, Place Georges-Teissier, BP 74, F29682 Roscoff Cedex, France. *corresponding author Fax: 33 2 98 29 23 24 ; E-mail: [email protected] Abstract: This review presents an account of the current knowledge concerning the endosymbiotic origin of plastids and mitochondria. The importance of algae as providing a large reservoir of diversified evolutionary models is emphasized. Several reviews describing the plastidial and mitochondrial genome organization and gene content have been published recently. Therefore we provide a survey of the different approaches that are used to investigate the evolution of organellar genomes since the endosymbiotic events. The importance of integrating population genetics concepts to understand better the global evolution of the cytoplasmically inherited organelles is especially emphasized. Résumé : Cette revue fait le point des connaissances actuelles concernant l’origine endosymbiotique des plastes et des mito- chondries en insistant plus particulièrement sur les données portant sur les algues. Ces organismes représentent en effet des lignées eucaryotiques indépendantes très diverses, et constituent ainsi un abondant réservoir de modèles évolutifs. L’organisation et le contenu en gènes des génomes plastidiaux et mitochondriaux chez les eucaryotes ont été détaillés exhaustivement dans plusieurs revues récentes. Nous présentons donc une synthèse des différentes approches utilisées pour comprendre l’évolution de ces génomes organitiques depuis l’événement endosymbiotique. En particulier nous soulignons l’importance des concepts de la génétique des populations pour mieux comprendre l’évolution des génomes à transmission cytoplasmique dans la cellule eucaryote. -

Localization of the Mitochondrial Ftsz Protein in a Dividing Mitochondrion Mitochondria Are Ubiquitous Organelles That Play Crit
C2001 The Japan Mendel Society Cytologia 66: 421-425, 2001 Localization of the Mitochondrial FtsZ Protein in a Dividing Mitochondrion Manabu Takahara*, Haruko Kuroiwa, Shin-ya Miyagishima, Toshiyuki Mori and Tsuneyoshi Kuroiwa Department of Biological Sciences, Graduate School of Science, University of Tokyo, Hongo, Tokyo 113-0033, Japan Accepted November 2, 2001 Summary FtsZ protein is essential for bacterial cell division, and is also involved in plastid and mitochondrial division. However, little is known of the function of FtsZ in the mitochondrial division process. Here, using electron microscopy, we revealed that the mitochondrial FtsZ (CmFtsZ 1) local- izes at the constricted isthmus of a dividing mitochondrion on the inner (matrix-side) surface of the mitochondrion. These results strongly suggest that the mitochondrial FtsZ acts as a ring structure on the inner surface of mitochondria. Key words Cyanidionschyzon merolae, FtsZ, FtsZ ring, mitochondria, mitochondrion-dividing ring (MD ring) . Mitochondria are ubiquitous organelles that play critical roles in respiration and ATP synthesis in almost all eukaryotic cells. Mitochondria are thought to have originated from a-proteobacteria through an endosymbiont event. Like bacteria, they multiply by the binary fission of pre-existing mitochondria. Although the role of mitochondria in respiration and ATP production is well under- stood, little is known about the proliferation of mitochondria in cells. In plastids, which also arose from prokaryotic endosymbionts, the ring structure that appears at the constricted isthmus of a dividing plastid (the plastid-dividing ring or PD ring) has been iden- tified as the plastid division apparatus (Mita et al. 1986). The PD ring is widespread among plants and algae, and consists of an outer (cytosolic) ring and an inner (stromal) ring in most species. -

Tobacco Chloroplast Ribosomes Contain a Homologue of E. Coli Ribosomal Protein L28
Volume 308. number 3, 258-260 FEBS 11439 August 1992 O 17,92 Federation of Earol.w.an Uioehemieal Societies 0014:~793/92/$~.00 Tobacco chloroplast ribosomes contain a homologue of E. coli ribosomal protein L28 Fumiaki Yokoi and Masahiro Sugiura Center for Gone l~exeareh. Nagoxa Univer#lO', Nagoya 464-01. $ttpa~# Received 12 May 1992; revi~d version received 7 July 1992 The ~nes for ribosomal proteins I~ and L33 constitute an opcron (rpm/~63 in ~'. chit. but in plant ~loroplasts L33 is en¢od~ by tl'm chloroplast DNA and L28 s~ms to be e~oded by the nuclear Ilenom¢, A 15 kDa protein was i~iated from the ~0 S subunit of ~bae.¢o chloroplast ribo~rrmc and its N.t©mfinal amino acid sequence was determined, A eDNA for this protein was cloned and analyzed, The eDNA enood¢~ a 151 amino add protein consistinil of a predicted transit.pcptide of 74 amino acids and a mature protein of 77 amino acids, Tlu~ mature protein is homolog,ou~ to E. cull L28, hen~ we named it chloroplast I..2tt (CL28). This is the first report on the preu:n~ ofnn B, ¢oli L.2Z.like protein in another ori~nism. Chloroplast Ribosomal protein: CI.28; Tobacco I. INTRODUCTION 2. MATERIAI.S AND METHODS 2.1. [xalalimt of rilmsm.,I prateitt CL28 Chloroplast ribosomes are 70 S in size similar to those Chloroplast ribosomal subunits *.,.'ere prepared from mature to. of E. coli and contain 3-5 different rgNAs and about bacco ieav~ (Hlrolia.tt ralmr~m!vat'. -

Structure and Function of the Archaeal Response Regulator Chey
Structure and function of the archaeal response PNAS PLUS regulator CheY Tessa E. F. Quaxa, Florian Altegoerb, Fernando Rossia, Zhengqun Lia, Marta Rodriguez-Francoc, Florian Krausd, Gert Bangeb,1, and Sonja-Verena Albersa,1 aMolecular Biology of Archaea, Faculty of Biology, University of Freiburg, 79104 Freiburg, Germany; bLandes-Offensive zur Entwicklung Wissenschaftlich- ökonomischer Exzellenz Center for Synthetic Microbiology & Faculty of Chemistry, Philipps-University-Marburg, 35043 Marburg, Germany; cCell Biology, Faculty of Biology, University of Freiburg, 79104 Freiburg, Germany; and dFaculty of Chemistry, Philipps-University-Marburg, 35043 Marburg, Germany Edited by Norman R. Pace, University of Colorado at Boulder, Boulder, CO, and approved December 13, 2017 (received for review October 2, 2017) Motility is a central feature of many microorganisms and provides display different swimming mechanisms. Counterclockwise ro- an efficient strategy to respond to environmental changes. Bacteria tation results in smooth swimming in well-characterized peritri- and archaea have developed fundamentally different rotary motors chously flagellated bacteria such as Escherichia coli, whereas enabling their motility, termed flagellum and archaellum, respec- rotation in the opposite direction results in tumbling. In contrast, tively. Bacterial motility along chemical gradients, called chemo- in other bacteria (i.e., Vibrio alginolyticus) and haloarchaea, taxis, critically relies on the response regulator CheY, which, when clockwise rotation results -

Interdependence Between Chloroplasts and Mitochondria in the Light and the Dark
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Elsevier - Publisher Connector Biochimica et Biophysica Acta 1366 (1998) 235^255 Review Interdependence between chloroplasts and mitochondria in the light and the dark Marcel H.N. Hoefnagel a, Owen K. Atkin b, Joseph T. Wiskich a;* a Department of Botany, University of Adelaide, Adelaide, SA 5005, Australia b Research School for Biological Sciences, Australian National University, Canberra, ACT 0200, Australia Received 21 April 1998; revised 3 June 1998; accepted 10 June 1998 ß 1998 Elsevier Science B.V. All rights reserved. Keywords: Chloroplast; Chlororespiration; Excess reductant; Metabolite exchange; Mitochondrion; Photosynthesis; Respiration Contents 1. Introduction .......................................................... 236 2. Interactions between organelles depends on metabolite exchange . .................. 236 2.1. ATP exchange ..................................................... 236 2.2. Transport of reducing equivalents across membranes . ....................... 237 2.3. Exchange of carbon compounds ....................................... 238 3. Respiration in the light . ................................................. 239 3.1. Does respiration continue in the light? . .................................. 239 3.2. Substrates for the mitochondria in the light . ............................ 240 3.3. ATP supply in the light: chloroplasts versus mitochondria . .................. 240 3.4. Adenylate control of respiration in the light . -

Mitosomes in Entamoeba Histolytica Contain a Sulfate Activation Pathway
Mitosomes in Entamoeba histolytica contain a sulfate activation pathway Fumika Mi-ichi, Mohammad Abu Yousuf, Kumiko Nakada-Tsukui, and Tomoyoshi Nozaki1 Department of Parasitology, National Institute of Infectious Diseases, Tokyo 162-8640, Japan Edited by Andrew Roger, Dalhousie University, Halifax, NS, Canada, and accepted by the Editorial Board October 19, 2009 (received for review June 25, 2009) Hydrogenosomes and mitosomes are mitochondrion-related or- and M. balamuthi lack the ISC system, and instead possess the ganelles in anaerobic/microaerophilic eukaryotes with highly re- nitrogen fixation (NIF) system, which is most likely derived from an duced and divergent functions. The full diversity of their content ancestral nitrogen fixing -proteobacterium by lateral gene transfer and function, however, has not been fully determined. To under- (22, 24). Only 5 proteins have been demonstrated in E. histolytica stand the central role of mitosomes in Entamoeba histolytica,a mitosomes: Cpn60 (8–10, 12), Cpn10 (13), mitochondrial Hsp70 parasitic protozoon that causes amoebic dysentery and liver ab- (11, 15), pyridine nucleotide transhydrogenase (PNT) (2, 8), and scesses, we examined the proteomic profile of purified mitosomes. mitochondria carrier family (MCF, ADP/ATP transporter) (14), Using 2 discontinuous Percoll gradient centrifugation and MS and the central role of mitosomes in E. histolytica remains unknown. analysis, we identified 95 putative mitosomal proteins. Immuno- Analysis of the genome of E. histolytica has not revealed any fluorescence assay showed that 3 proteins involved in sulfate additional information regarding the function of mitosomes and activation, ATP sulfurylase, APS kinase, and inorganic pyrophos- thus, a proteomic analysis of mitosomes seems to be the best phatase, as well as sodium/sulfate symporter, involved in sulfate approach to understand its structure and function (1, 2). -

A Novel ADP/ATP Transporter in the Mitosome of the Microaerophilic Human Parasite Entamoeba Histolytica
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Elsevier - Publisher Connector Current Biology, Vol. 15, 737–742, April 26, 2005, ©2005 Elsevier Ltd All rights reserved. DOI 10.1016/j.cub.2005.02.068 A Novel ADP/ATP Transporter in the Mitosome of the Microaerophilic Human Parasite Entamoeba histolytica Ka Wai Chan,1,5 Dirk-Jan Slotboom,1,5 Sian Cox,3 ses confirm that the Entamoeba ADP/ATP carrier is T. Martin Embley,2,* Olivier Fabre,1 distinct from archetypal mitochondrial ADP/ATP carri- Mark van der Giezen,3,6 Marilyn Harding,1 ers, an observation that is supported by its different David S. Horner,4 Edmund R.S. Kunji,1,* substrate and inhibitor specificity. Because many Gloria León-Avila,3,7 and Jorge Tovar3 functions of yeast and human mitochondria rely on 1Dunn Human Nutrition Unit solutes transported by specialized members of this Medical Research Council family, the Entamoeba mitosome must contain only Hills Road a small subset of these processes requiring adenine Cambridge CB2 2XY nucleotide exchange. United Kingdom 2 School of Biology The Devonshire Building Results and Discussion University of Newcastle upon Tyne Newcastle NE1 7RU Identification of an MCF Homolog United Kingdom on the Entamoeba histolytica Genome 3 School of Biological Sciences We identified a homolog of the mitochondrial carrier Royal Holloway family (MCF) on the Entamoeba histolytica genome (TIGR) University of London with BLAST searches. The translated protein contains a Egham, Surrey TW20 0EX tripartite structure [8], signature motifs (IPR001993), and United Kingdom other features typical of MCF members (Figure 1A). -

Crosstalk and the Evolution of Specificity in Two-Component Signaling
Corrections BIOPHYSICS AND COMPUTATIONAL BIOLOGY PLANT BIOLOGY Correction for “Crosstalk and the evolution of specificity in two- Correction for “Differential processing of Arabidopsis ubiquitin- component signaling,” by Michael A. Rowland and Eric J. Deeds, like Atg8 autophagy proteins by Atg4 cysteine proteases,” by which appeared in issue 15, April 15, 2014, of Proc Natl Acad Sci Jongchan Woo, Eunsook Park, and S. P. Dinesh-Kumar, which USA (111:5550–5555; first published March 31, 2014; 10.1073/ appeared in issue 2, January 14, 2014, of Proc Natl Acad Sci pnas.1317178111). USA (111:863–868; first published December 30, 2013; 10.1073/ The authors note that ref. 4, “Huynh TN, Stewart V (2011) pnas.1318207111). Negative control in two-component signal transduction by trans- The authors note that the following statement should be mitter phosphatase activity. Mol Microbiol 82(2):275–286.” should added to the Acknowledgments: “National Science Foundation instead appear as “Ray JC, Igoshin OA (2010) Adaptable func- Grant NSF-IOS-1258135 funded to Dr. Georgia Drakakaki sup- tionality of transcriptional feedback in bacterial two-component ported Eunsook Park.” systems. PLoS Comput Biol 6(2):e1000676.” www.pnas.org/cgi/doi/10.1073/pnas.1409947111 www.pnas.org/cgi/doi/10.1073/pnas.1408294111 CORRECTIONS www.pnas.org PNAS | June 24, 2014 | vol. 111 | no. 25 | 9325 Downloaded by guest on September 29, 2021 Crosstalk and the evolution of specificity in two-component signaling Michael A. Rowlanda and Eric J. Deedsa,b,1 aCenter for Bioinformatics and bDepartment of Molecular Biosciences, University of Kansas, Lawrence, KS 66047 Edited by Thomas J.