Nail Acidification Vs. Amorolfine in the Local Management of Onychomycosis: a Comparative, Prospective, Randomized, Blinded Trial
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

New Insights Into the Effect of Amorolfine Nail Lacquer
Review article New insights into the effect of amorolfine nail lacquer C. Flagothier, C. Pie´ rard-Franchimont and G. E. Pie´ rard Department of Dermatopathology, University Hospital of Lie`ge, Lie`ge, Belgium Summary Despite improvements in antifungal strategies, the outcome of treating onychomycoses often remains uncertain. Several factors account for treatment failure, of which the pharmacokinetics and pharmacodynamics of the antifungal are of importance. The taxonomic nature and ungual location of the fungus cannot be neglected, besides the type of nail and its growth rate. In addition, the biological cycle of the fungus and the metabolic activity of the pathogen likely play a marked influence in drug response. The presence of natural antimicrobial peptides in the nail is also probably a key feature controlling the cure rates. There are many outstanding publications that cover the full spectrum of the field. The purpose of this review is to put in perspective some facets of activity of the topical treatment using amorolfine nail laquer. The antifungal activity of the drug is likely less pronounced in onychomycosis than that expected from conventional in vitro studies. However, the nail laquer formulation should reduce the propensity to form antifungal-resistant spores and limit the risk of reinfection. Key words: amorolfine, antifungal, fungus, onychomycosis, spore. Topical treatments are often considered to be less Introduction efficacious than current oral treatments. However, some During the last 2 decades, the efficacy of treating topical formulations may provide effects that cannot be onychomycoses has been considerably improved by achieved by other treatments. In discussing the treat- the introduction of new generations of potent antifun- ment of onychomycosis, it should not be forgotten that gals. -

The Management of Common Skin Conditions in General Practice
Management of Common Skin Conditions In General Practice including the “red rash made easy” © Arroll, Fishman & Oakley, Department of General Practice and Primary Health Care University of Auckland, Tamaki Campus Reviewed by Hon A/Prof Amanda Oakley - 2019 http://www.dermnetnz.org Management of Common Skin Conditions In General Practice Contents Page Derm Map 3 Classic location: infants & children 4 Classic location: adults 5 Dermatology terminology 6 Common red rashes 7 Other common skin conditions 12 Common viral infections 14 Common bacterial infections 16 Common fungal infections 17 Arthropods 19 Eczema/dermatitis 20 Benign skin lesions 23 Skin cancers 26 Emergency dermatology 28 Clinical diagnosis of melanoma 31 Principles of diagnosis and treatment 32 Principles of treatment of eczema 33 Treatment sequence for psoriasis 34 Topical corticosteroids 35 Combination topical steroid + antimicrobial 36 Safety with topical corticosteroids 36 Emollients 37 Antipruritics 38 For further information, refer to: http://www.dermnetnz.org And http://www.derm-master.com 2 © Arroll, Fishman & Oakley, Department of General Practice and Primary Health Care, University of Auckland, Tamaki Campus. Management of Common Skin Conditions In General Practice DERM MAP Start Is the patient sick ? Yes Rash could be an infection or a drug eruption? No Insect Bites – Crop of grouped papules with a central blister or scab. Is the patient in pain or the rash Yes Infection: cellulitis / erysipelas, impetigo, boil is swelling, oozing or crusting? / folliculitis, herpes simplex / zoster. Urticaria – Smooth skin surface with weals that evolve in minutes to hours. No Is the rash in a classic location? Yes See our classic location chart . -

Antimicrobial Resistance Benchmark 2020 Antimicrobial Resistance Benchmark 2020
First independent framework for assessing pharmaceutical company action Antimicrobial Resistance Benchmark 2020 Antimicrobial Resistance Benchmark 2020 ACKNOWLEDGEMENTS The Access to Medicine Foundation would like to thank the following people and organisations for their contributions to this report.1 FUNDERS The Antimicrobial Resistance Benchmark research programme is made possible with financial support from UK AID and the Dutch Ministry of Health, Welfare and Sport. Expert Review Committee Research Team Reviewers Hans Hogerzeil - Chair Gabrielle Breugelmans Christine Årdal Gregory Frank Fatema Rafiqi Karen Gallant Nina Grundmann Adrián Alonso Ruiz Hans Hogerzeil Magdalena Kettis Ruth Baron Hitesh Hurkchand Joakim Larsson Dulce Calçada Joakim Larsson Marc Mendelson Moska Hellamand Marc Mendelson Margareth Ndomondo-Sigonda Kevin Outterson Katarina Nedog Sarah Paulin (Observer) Editorial Team Andrew Singer Anna Massey Deirdre Cogan ACCESS TO MEDICINE FOUNDATION Rachel Jones The Access to Medicine Foundation is an independent Emma Ross non-profit organisation based in the Netherlands. It aims to advance access to medicine in low- and middle-income Additional contributors countries by stimulating and guiding the pharmaceutical Thomas Collin-Lefebvre industry to play a greater role in improving access to Alex Kong medicine. Nestor Papanikolaou Address Contact Naritaweg 227-A For more information about this publication, please contact 1043 CB, Amsterdam Jayasree K. Iyer, Executive Director The Netherlands [email protected] +31 (0) 20 215 35 35 www.amrbenchmark.org 1 This acknowledgement is not intended to imply that the individuals and institutions referred to above endorse About the cover: Young woman from the Antimicrobial Resistance Benchmark methodology, Brazil, where 40%-60% of infections are analyses or results. -

Oral Antifungals Month/Year of Review: July 2015 Date of Last
© Copyright 2012 Oregon State University. All Rights Reserved Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35 Salem, Oregon 97301-1079 Phone 503-947-5220 | Fax 503-947-1119 Class Update with New Drug Evaluation: Oral Antifungals Month/Year of Review: July 2015 Date of Last Review: March 2013 New Drug: isavuconazole (a.k.a. isavunconazonium sulfate) Brand Name (Manufacturer): Cresemba™ (Astellas Pharma US, Inc.) Current Status of PDL Class: See Appendix 1. Dossier Received: Yes1 Research Questions: Is there any new evidence of effectiveness or safety for oral antifungals since the last review that would change current PDL or prior authorization recommendations? Is there evidence of superior clinical cure rates or morbidity rates for invasive aspergillosis and invasive mucormycosis for isavuconazole over currently available oral antifungals? Is there evidence of superior safety or tolerability of isavuconazole over currently available oral antifungals? • Is there evidence of superior effectiveness or safety of isavuconazole for invasive aspergillosis and invasive mucormycosis in specific subpopulations? Conclusions: There is low level evidence that griseofulvin has lower mycological cure rates and higher relapse rates than terbinafine and itraconazole for adult 1 onychomycosis.2 There is high level evidence that terbinafine has more complete cure rates than itraconazole (55% vs. 26%) for adult onychomycosis caused by dermatophyte with similar discontinuation rates for both drugs.2 There is low -

Evaluation of the Drug Treatment and Persistence of Onychomycosis
Review TheScientificWorldJOURNAL (2004) 4, 760–777 ISSN 1537-744X; DOI 10.1100/tsw.2004.134 Evaluation of the Drug Treatment and Persistence of Onychomycosis Andrew W. Campbell1, Ebere C. Anyanwu2,*, and Mohammed Morad3 1Medical Center for Immune and Toxic Disorders, 20510 Oakhurst Drive, Suite 200, Spring TX; 2Neurosciences Research, Cahers Inc., 8787 Shenandoah Park Drive, Suite 122, Conroe, Houston, 77385 TX; 3Clalit Health Services and Division of Community Health, Department of Family Medicine, Ben Gurion University, Beer-Sheva, Israel E-mail: [email protected] Received August 1, 2004; Revised August 18, 2004; Accepted August 19, 2004; Published August 31, 2004 Onychomycosis is a common nail disease responsible for approximately 50% of diseases of the nail. It occurs more in the elderly, though several cases have been reported among children. Several factors influence, such as climate, geography, and migration. The two dermatophytes most commonly implicated in onychomycosis are Trichophyton rubrum and T. mentagrophytes, accounting for more than 90% of onychomycoses. Nonetheless, several other toxigenic molds have been implicated. For convenience, onychomycosis is divided into four major clinical presentations: distal subungal, which is the most common form of the disease; proximal subungal, which is the most common form found in patients with human immunodeficiency virus infection; superficial; and total dystrophic onychomycosis. Epidemiology of onychomycosis in adults and children is evaluated and the most common clinical symptoms addressed. Although the risk factors are discussed, the multifactorial nature of onychomycosis makes this inexhaustible. The diagnosis and treatments are difficult and the choice of appropriate antifungal drugs complex and require the knowledge of the chemical structures of the metabolites of the molds that cause onychomycosis and their interaction with the antifungal drugs. -

Estonian Statistics on Medicines 2016 1/41
Estonian Statistics on Medicines 2016 ATC code ATC group / Active substance (rout of admin.) Quantity sold Unit DDD Unit DDD/1000/ day A ALIMENTARY TRACT AND METABOLISM 167,8985 A01 STOMATOLOGICAL PREPARATIONS 0,0738 A01A STOMATOLOGICAL PREPARATIONS 0,0738 A01AB Antiinfectives and antiseptics for local oral treatment 0,0738 A01AB09 Miconazole (O) 7088 g 0,2 g 0,0738 A01AB12 Hexetidine (O) 1951200 ml A01AB81 Neomycin+ Benzocaine (dental) 30200 pieces A01AB82 Demeclocycline+ Triamcinolone (dental) 680 g A01AC Corticosteroids for local oral treatment A01AC81 Dexamethasone+ Thymol (dental) 3094 ml A01AD Other agents for local oral treatment A01AD80 Lidocaine+ Cetylpyridinium chloride (gingival) 227150 g A01AD81 Lidocaine+ Cetrimide (O) 30900 g A01AD82 Choline salicylate (O) 864720 pieces A01AD83 Lidocaine+ Chamomille extract (O) 370080 g A01AD90 Lidocaine+ Paraformaldehyde (dental) 405 g A02 DRUGS FOR ACID RELATED DISORDERS 47,1312 A02A ANTACIDS 1,0133 Combinations and complexes of aluminium, calcium and A02AD 1,0133 magnesium compounds A02AD81 Aluminium hydroxide+ Magnesium hydroxide (O) 811120 pieces 10 pieces 0,1689 A02AD81 Aluminium hydroxide+ Magnesium hydroxide (O) 3101974 ml 50 ml 0,1292 A02AD83 Calcium carbonate+ Magnesium carbonate (O) 3434232 pieces 10 pieces 0,7152 DRUGS FOR PEPTIC ULCER AND GASTRO- A02B 46,1179 OESOPHAGEAL REFLUX DISEASE (GORD) A02BA H2-receptor antagonists 2,3855 A02BA02 Ranitidine (O) 340327,5 g 0,3 g 2,3624 A02BA02 Ranitidine (P) 3318,25 g 0,3 g 0,0230 A02BC Proton pump inhibitors 43,7324 A02BC01 Omeprazole -

Fungal Infections (Mycoses): Dermatophytoses (Tinea, Ringworm)
Editorial | Journal of Gandaki Medical College-Nepal Fungal Infections (Mycoses): Dermatophytoses (Tinea, Ringworm) Reddy KR Professor & Head Microbiology Department Gandaki Medical College & Teaching Hospital, Pokhara, Nepal Medical Mycology, a study of fungal epidemiology, ecology, pathogenesis, diagnosis, prevention and treatment in human beings, is a newly recognized discipline of biomedical sciences, advancing rapidly. Earlier, the fungi were believed to be mere contaminants, commensals or nonpathogenic agents but now these are commonly recognized as medically relevant organisms causing potentially fatal diseases. The discipline of medical mycology attained recognition as an independent medical speciality in the world sciences in 1910 when French dermatologist Journal of Raymond Jacques Adrien Sabouraud (1864 - 1936) published his seminal treatise Les Teignes. This monumental work was a comprehensive account of most of then GANDAKI known dermatophytes, which is still being referred by the mycologists. Thus he MEDICAL referred as the “Father of Medical Mycology”. COLLEGE- has laid down the foundation of the field of Medical Mycology. He has been aptly There are significant developments in treatment modalities of fungal infections NEPAL antifungal agent available. Nystatin was discovered in 1951 and subsequently and we have achieved new prospects. However, till 1950s there was no specific (J-GMC-N) amphotericin B was introduced in 1957 and was sanctioned for treatment of human beings. In the 1970s, the field was dominated by the azole derivatives. J-GMC-N | Volume 10 | Issue 01 developed to treat fungal infections. By the end of the 20th century, the fungi have Now this is the most active field of interest, where potential drugs are being January-June 2017 been reported to be developing drug resistance, especially among yeasts. -

Nails: Tales, Fails and What Prevails in Treating Onychomycosis
J. Hibler, D.O. OhioHealth - O’Bleness Memorial Hospital, Athens, Ohio AOCD Annual Conference Orlando, Florida 10.18.15 A) Onychodystrophy B) Onychogryphosis C)“Question Onychomycosis dogma” – Michael Conroy, MD D) All the above E) None of the above Nail development begins at 8-10 weeks EGA Complete by 5th month Keratinization ~11 weeks No granular layer Nail plate growth: Fingernails 3 mm/month, toenails 1 mm/month Faster in summer or winter? Summer! Index finger or 5th digit nail grows faster? Index finger! Faster growth to middle or lateral edge of each nail? Lateral! Elkonyxis Mee’s lines Aka leukonychia striata Arsenic poisoning Trauma Medications Illness Psoriasis flare Muerhrcke’s bands Hypoalbuminemia Chemotherapy Half & half nails Aka Lindsay’s nails Chronic renal disease Terry’s nails Liver failure, Cirrhosis Malnutrition Diabetes Cardiovascular disease True or False: Onychomycosis = Tinea Unguium? FALSE. Onychomycosis: A fungal disease of the nails (all causes) Dermatophytes, yeasts, molds Tinea unguium: A fungal disease of nail caused by dermatophyte fungi Onychodystrophy ≠ onychomycosis Accounts for up to 50% of all nail disorders Prevalence; 14-28% of > 60 year-olds Variety of subtypes; know them! Sequelae What is the most common cause of onychomycosis? A) Epidermophyton floccosum B) Microsporum spp C) Trichophyton mentagrophytes D) Trichophyton rubrum -Account for ~90% of infections Dermatophytes Trichophyton rubrum Trichophyton mentagrophytes Trichophyton tonsurans, Microsporum canis, Epidermophyton floccosum Nondermatophyte molds Acremonium spp, Fusarium spp Scopulariopsis spp, Sytalidium spp, Aspergillus spp Yeast Candida parapsilosis Candida albicans Candida spp Distal/lateral subungal Proximal subungual onychomycosis onychomycosis (DLSO) (PSO) Most common; T. rubrum Often in immunosuppressed patients T. -

Drug Consumption in Current Year (Period 201901
Page 1 Drug consumption in current year (Period 202001 - 202012) Wholesale ATC code Subgroup or chemical substance DDD/1000 inhab./day Hospital % Change % price/1000 € Hospital % Change % A ALIMENTARY TRACT AND METABOLISM 323,80 3 4 321 589 7 4 A01 STOMATOLOGICAL PREPARATIONS 14,28 4 12 2 090 9 8 A01A STOMATOLOGICAL PREPARATIONS 14,28 4 12 2 090 9 8 A01AA Caries prophylactic agents 11,90 3 14 663 8 9 A01AA01 sodium fluoride 11,90 3 14 610 8 10 A01AA03 olaflur - - - 53 1 -2 A01AB Antiinfectives for local oral treatment 2,36 8 2 1 266 10 15 A01AB03 chlorhexidine 2,02 6 -3 930 6 5 A01AB11 various 0,33 21 57 335 21 55 A01AB22 doxycycline - - - 0 - -100 A01AC Corticosteroids for local oral treatment - - - 113 1 -26 A01AC01 triamcinolone - - - 113 1 -26 A01AD Other agents for local oral treatment 0,02 0 -28 49 0 -32 A01AD02 benzydamine 0,02 0 -28 49 0 -32 A02 DRUGS FOR ACID RELATED DISORDERS 73,05 3 3 30 885 4 -5 A02A ANTACIDS 2,23 1 1 3 681 1 3 A02AA Magnesium compounds 0,07 22 -7 141 22 -7 A02AA04 magnesium hydroxide 0,07 22 -7 141 22 -7 A02AD Combinations and complexes of aluminium, 2,17 0 1 3 539 0 4 calcium and magnesium compounds A02AD01 ordinary salt combinations 2,17 0 1 3 539 0 4 A02B DRUGS FOR PEPTIC ULCER AND 70,82 3 3 27 205 5 -7 GASTRO-OESOPHAGEAL REFLUX DISEASE (GORD) A02BA H2-receptor antagonists 0,17 7 -77 551 10 -29 A02BA02 ranitidine 0,00 1 -100 1 1 -100 A02BA03 famotidine 0,16 7 48 550 10 43 A02BB Prostaglandins 0,04 62 55 80 62 55 A02BB01 misoprostol 0,04 62 55 80 62 55 A02BC Proton pump inhibitors 69,26 3 4 23 531 4 -8 -

OUH Formulary Approved for Use in Breast Surgery
Oxford University Hospitals NHS Foundation Trust Formulary FORMULARY (Y): the medicine can be used as per its licence. RESTRICTED FORMULARY (R): the medicine can be used as per the agreed restriction. NON-FORMULARY (NF): the medicine is not on the formulary and should not be used unless exceptional approval has been obtained from MMTC. UNLICENSED MEDICINE – RESTRICTED FORMULARY (UNR): the medicine is unlicensed and can be used as per the agreed restriction. SPECIAL MEDICINE – RESTRICTED FORMULARY (SR): the medicine is a “special” (unlicensed) and can be used as per the agreed restriction. EXTEMPORANEOUS PREPARATION – RESTRICTED FORMULARY (EXTR): the extemporaneous preparation (unlicensed) can be prepared and used as per the agreed restriction. UNLICENSED MEDICINE – NON-FORMULARY (UNNF): the medicine is unlicensed and is not on the formulary. It should not be used unless exceptional approval has been obtained from MMTC. SPECIAL MEDICINE – NON-FORMULARY (SNF): the medicine is a “special” (unlicensed) and is not on the formulary. It should not be used unless exceptional approval has been obtained from MMTC. EXTEMPORANEOUS PREPARATION – NON-FORMULARY (EXTNF): the extemporaneous preparation (unlicensed) cannot be prepared and used unless exceptional approval has been obtained from MMTC. CLINICAL TRIALS (C): the medicine is clinical trial material and is not for clinical use. NICE TECHNOLOGY APPRAISAL (NICETA): the medicine has received a positive appraisal from NICE. It will be available on the formulary from the day the Technology Appraisal is published. Prescribers who wish to treat patients who meet NICE criteria, will have access to these medicines from this date. However, these medicines will not be part of routine practice until a NICE TA Implementation Plan has been presented and approved by MMTC (when the drug will be given a Restricted formulary status). -

Drug Consumption at Wholesale Prices in 2017 - 2020
Page 1 Drug consumption at wholesale prices in 2017 - 2020 2020 2019 2018 2017 Wholesale Hospit. Wholesale Hospit. Wholesale Hospit. Wholesale Hospit. ATC code Subgroup or chemical substance price/1000 € % price/1000 € % price/1000 € % price/1000 € % A ALIMENTARY TRACT AND METABOLISM 321 590 7 309 580 7 300 278 7 295 060 8 A01 STOMATOLOGICAL PREPARATIONS 2 090 9 1 937 7 1 910 7 2 128 8 A01A STOMATOLOGICAL PREPARATIONS 2 090 9 1 937 7 1 910 7 2 128 8 A01AA Caries prophylactic agents 663 8 611 11 619 12 1 042 11 A01AA01 sodium fluoride 610 8 557 12 498 15 787 14 A01AA03 olaflur 53 1 54 1 50 1 48 1 A01AA51 sodium fluoride, combinations - - - - 71 1 206 1 A01AB Antiinfectives for local oral treatment 1 266 10 1 101 6 1 052 6 944 6 A01AB03 chlorhexidine 930 6 885 7 825 7 706 7 A01AB11 various 335 21 216 0 227 0 238 0 A01AB22 doxycycline - - 0 100 0 100 - - A01AC Corticosteroids for local oral treatment 113 1 153 1 135 1 143 1 A01AC01 triamcinolone 113 1 153 1 135 1 143 1 A01AD Other agents for local oral treatment 49 0 72 0 104 0 - - A01AD02 benzydamine 49 0 72 0 104 0 - - A02 DRUGS FOR ACID RELATED DISORDERS 30 885 4 32 677 4 35 102 5 37 644 7 A02A ANTACIDS 3 681 1 3 565 1 3 357 1 3 385 1 A02AA Magnesium compounds 141 22 151 22 172 22 155 19 A02AA04 magnesium hydroxide 141 22 151 22 172 22 155 19 A02AD Combinations and complexes of aluminium, 3 539 0 3 414 0 3 185 0 3 231 0 calcium and magnesium compounds A02AD01 ordinary salt combinations 3 539 0 3 414 0 3 185 0 3 231 0 A02B DRUGS FOR PEPTIC ULCER AND 27 205 5 29 112 4 31 746 5 34 258 8 -
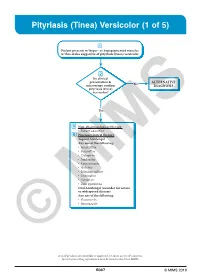
Pityriasis (Tinea) Versicolor (1 of 5)
Pityriasis (Tinea) Versicolor (1 of 5) 1 Patient presents w/ hyper- or hypopigmented macules w/ fine scales suggestive of pityriasis (tinea) versicolor 2 Do clinical presentation & No ALTERNATIVE microscopy confirm DIAGNOSIS pityriasis (tinea) versicolor? Yes A Non-pharmacological therapy • Patient education B Pharmacological therapy Topical Antifungal Any one of the following: • Amorolfi ne • Butenafi ne • Ciclopirox • Imidazoles • Ketoconazole • Naftifi ne • Selenium sulfi de • Terbinafi ne • Tolnaftate • Zinc pyrithione Oral Antifungal (consider for severe or widespread disease) Any one of the following: • Fluconazole • Itraconazole © MIMS Not all products are available or approved for above use in all countries. Specifi c prescribing information may be found in the latest MIMS. B307 © MIMS 2019 Pityriasis (Tinea) Versicolor (2 of 5) 1 EVALUATION Pityriasis (tinea) versicolor is a common, benign, superfi cial fungal infection localized to the stratum corneum • Caused by lipophilic yeasts, Malassezia species, part of the normal fl ora of the human skin Clinical Presentation • May present as chronic or recurrent infection & may occur in healthy individuals - More common in summer than winter months • Predominates in young adults when the sebaceous glands are most active • Presents w/ multiple well-demarcated macules or patches & fi nely scaled plaques w/ hypopigmentation or hyperpigmentation, hence the term “versicolor” • Tends to be asymptomatic & is mainly a cosmetic concern but pruritus may or may not be present • Usually found on the upper trunk,