Contributions of De Novo Variants to Systemic Lupus Erythematosus
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
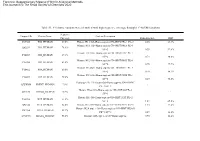
Table S1. 49 Histone Variants Were Identified with High Sequence Coverage Through LC-MS/MS Analysis Electronic Supplementary
Electronic Supplementary Material (ESI) for Analytical Methods. This journal is © The Royal Society of Chemistry 2020 Table S1. 49 histone variants were identified with high sequence coverage through LC-MS/MS analysis Sequence Uniprot IDs Protein Name Protein Description Coverage Ratio E2+/E2- RSD P07305 H10_HUMAN 67.5% Histone H1.0 OS=Homo sapiens GN=H1F0 PE=1 SV=3 4.85 23.3% Histone H1.1 OS=Homo sapiens GN=HIST1H1A PE=1 Q02539 H11_HUMAN 74.4% SV=3 0.35 92.6% Histone H1.2 OS=Homo sapiens GN=HIST1H1C PE=1 P16403 H12_HUMAN 67.1% SV=2 0.73 80.6% Histone H1.3 OS=Homo sapiens GN=HIST1H1D PE=1 P16402 H13_HUMAN 63.8% SV=2 0.75 77.7% Histone H1.4 OS=Homo sapiens GN=HIST1H1E PE=1 P10412 H14_HUMAN 69.0% SV=2 0.70 80.3% Histone H1.5 OS=Homo sapiens GN=HIST1H1B PE=1 P16401 H15_HUMAN 79.6% SV=3 0.29 98.3% Testis-specific H1 histone OS=Homo sapiens GN=H1FNT Q75WM6 H1FNT_HUMAN 7.8% \ \ PE=2 SV=3 Histone H1oo OS=Homo sapiens GN=H1FOO PE=2 Q8IZA3 H1FOO_HUMAN 5.2% \ \ SV=1 Histone H1t OS=Homo sapiens GN=HIST1H1T PE=2 P22492 H1T_HUMAN 31.4% SV=4 1.42 65.0% Q92522 H1X_HUMAN 82.6% Histone H1x OS=Homo sapiens GN=H1FX PE=1 SV=1 1.15 33.2% Histone H2A type 1 OS=Homo sapiens GN=HIST1H2AG P0C0S8 H2A1_HUMAN 99.2% PE=1 SV=2 0.57 26.8% Q96QV6 H2A1A_HUMAN 58.0% Histone H2A type 1-A OS=Homo sapiens 0.90 11.2% GN=HIST1H2AA PE=1 SV=3 Histone H2A type 1-B/E OS=Homo sapiens P04908 H2A1B_HUMAN 99.2% GN=HIST1H2AB PE=1 SV=2 0.92 30.2% Histone H2A type 1-C OS=Homo sapiens Q93077 H2A1C_HUMAN 100.0% GN=HIST1H2AC PE=1 SV=3 0.76 27.6% Histone H2A type 1-D OS=Homo sapiens P20671 -

UNIVERSITY of CALIFORNIA, SAN DIEGO Functional Analysis of Sall4
UNIVERSITY OF CALIFORNIA, SAN DIEGO Functional analysis of Sall4 in modulating embryonic stem cell fate A dissertation submitted in partial satisfaction of the requirements for the degree Doctor of Philosophy in Molecular Pathology by Pei Jen A. Lee Committee in charge: Professor Steven Briggs, Chair Professor Geoff Rosenfeld, Co-Chair Professor Alexander Hoffmann Professor Randall Johnson Professor Mark Mercola 2009 Copyright Pei Jen A. Lee, 2009 All rights reserved. The dissertation of Pei Jen A. Lee is approved, and it is acceptable in quality and form for publication on microfilm and electronically: ______________________________________________________________ ______________________________________________________________ ______________________________________________________________ ______________________________________________________________ Co-Chair ______________________________________________________________ Chair University of California, San Diego 2009 iii Dedicated to my parents, my brother ,and my husband for their love and support iv Table of Contents Signature Page……………………………………………………………………….…iii Dedication…...…………………………………………………………………………..iv Table of Contents……………………………………………………………………….v List of Figures…………………………………………………………………………...vi List of Tables………………………………………………….………………………...ix Curriculum vitae…………………………………………………………………………x Acknowledgement………………………………………………….……….……..…...xi Abstract………………………………………………………………..…………….....xiii Chapter 1 Introduction ..…………………………………………………………………………….1 Chapter 2 Materials and Methods……………………………………………………………..…12 -

The Human Dcn1-Like Protein DCNL3 Promotes Cul3 Neddylation at Membranes
The human Dcn1-like protein DCNL3 promotes Cul3 neddylation at membranes Nathalie Meyer-Schallera, Yang-Chieh Choub,c, Izabela Sumaraa, Dale D. O. Martind, Thimo Kurza, Nadja Kathedera, Kay Hofmanne, Luc G. Berthiaumed, Frank Sicherib,c, and Matthias Petera,1 aInstitute of Biochemistry, Eidgeno¨ssiche Technische Hochschule, 8093 Zurich, Switzerland; bCenter for Systems Biology, Samuel Lunenfeld Research Institute, Toronto, ON, Canada M5G 1X5; cDepartment of Molecular Genetics, University of Toronto, Toronto, ON, Canada M5S 1A8; dDepartment of Cell Biology, University of Alberta, Edmonton, AB, Canada T6G 2H7; and eBioinformatics Group, Miltenyi Biotec, 51429 Bergisch-Gladbach, Germany Edited by Michael Rape, University of California, Berkeley, CA, and accepted by the Editorial Board June 9, 2009 (received for review December 9, 2008) Cullin (Cul)-based E3 ubiquitin ligases are activated through the enzyme and promotes Nedd8 conjugation through formation of attachment of Nedd8 to the Cul protein. In yeast, Dcn1 (defective this complex (14, 15). Human cells harbor 5 Dcn1-like proteins in Cul neddylation 1 protein) functions as a scaffold-like Nedd8 termed DCNL1–DCNL5 (also named DCUN1D 1–5 for defec- E3-ligase by interacting with its Cul substrates and the Nedd8 E2 tive in Cul neddylation 1 domain-containing protein 1–5) (Fig. Ubc12. Human cells express 5 Dcn1-like (DCNL) proteins each S1). These DCNLs have distinct amino-terminal domains, but containing a C-terminal potentiating neddylation domain but dis- share a conserved C-terminal potentiating neddylation (PONY) tinct amino-terminal extensions. Although the UBA-containing domain, which in yeast Dcn1 is necessary and sufficient for Cul DCNL1 and DCNL2 are likely functional homologues of yeast Dcn1, neddylation in vivo and in vitro (14). -

An Integrative, Genomic, Transcriptomic and Network-Assisted
Yan et al. BMC Medical Genomics 2020, 13(Suppl 5):39 https://doi.org/10.1186/s12920-020-0675-4 RESEARCH Open Access An integrative, genomic, transcriptomic and network-assisted study to identify genes associated with human cleft lip with or without cleft palate Fangfang Yan1†, Yulin Dai1†, Junichi Iwata2,3, Zhongming Zhao1,4,5* and Peilin Jia1* From The International Conference on Intelligent Biology and Medicine (ICIBM) 2019 Columbus, OH, USA. 9-11 June 2019 Abstract Background: Cleft lip with or without cleft palate (CL/P) is one of the most common congenital human birth defects. A combination of genetic and epidemiology studies has contributed to a better knowledge of CL/P-associated candidate genes and environmental risk factors. However, the etiology of CL/P remains not fully understood. In this study, to identify new CL/P-associated genes, we conducted an integrative analysis using our in-house network tools, dmGWAS [dense module search for Genome-Wide Association Studies (GWAS)] and EW_dmGWAS (Edge-Weighted dmGWAS), in a combination with GWAS data, the human protein-protein interaction (PPI) network, and differential gene expression profiles. Results: A total of 87 genes were consistently detected in both European and Asian ancestries in dmGWAS. There were 31.0% (27/87) showed nominal significance with CL/P (gene-based p < 0.05), with three genes showing strong association signals, including KIAA1598, GPR183,andZMYND11 (p <1×10− 3). In EW_dmGWAS, we identified 253 and 245 module genes associated with CL/P for European ancestry and the Asian ancestry, respectively. Functional enrichment analysis demonstrated that these genes were involved in cell adhesion, protein localization to the plasma membrane, the regulation of the apoptotic signaling pathway, and other pathological conditions. -

Neddylation: a Novel Modulator of the Tumor Microenvironment Lisha Zhou1,2*†, Yanyu Jiang3†, Qin Luo1, Lihui Li1 and Lijun Jia1*
Zhou et al. Molecular Cancer (2019) 18:77 https://doi.org/10.1186/s12943-019-0979-1 REVIEW Open Access Neddylation: a novel modulator of the tumor microenvironment Lisha Zhou1,2*†, Yanyu Jiang3†, Qin Luo1, Lihui Li1 and Lijun Jia1* Abstract Neddylation, a post-translational modification that adds an ubiquitin-like protein NEDD8 to substrate proteins, modulates many important biological processes, including tumorigenesis. The process of protein neddylation is overactivated in multiple human cancers, providing a sound rationale for its targeting as an attractive anticancer therapeutic strategy, as evidence by the development of NEDD8-activating enzyme (NAE) inhibitor MLN4924 (also known as pevonedistat). Neddylation inhibition by MLN4924 exerts significantly anticancer effects mainly by triggering cell apoptosis, senescence and autophagy. Recently, intensive evidences reveal that inhibition of neddylation pathway, in addition to acting on tumor cells, also influences the functions of multiple important components of the tumor microenvironment (TME), including immune cells, cancer-associated fibroblasts (CAFs), cancer-associated endothelial cells (CAEs) and some factors, all of which are crucial for tumorigenesis. Here, we briefly summarize the latest progresses in this field to clarify the roles of neddylation in the TME, thus highlighting the overall anticancer efficacy of neddylaton inhibition. Keywords: Neddylation, Tumor microenvironment, Tumor-derived factors, Cancer-associated fibroblasts, Cancer- associated endothelial cells, Immune cells Introduction Overall, binding of NEDD8 molecules to target proteins Neddylation is a reversible covalent conjugation of an can affect their stability, subcellular localization, conform- ubiquitin-like molecule NEDD8 (neuronal precursor ation and function [4]. The best-characterized substrates cell-expressed developmentally down-regulated protein of neddylation are the cullin subunits of Cullin-RING li- 8) to a lysine residue of the substrate protein [1, 2]. -

And CAND1-Dependent Remodelling of the Budding Yeast SCF Complex
ARTICLE Received 31 Jan 2013 | Accepted 20 Feb 2013 | Published 27 Mar 2013 DOI: 10.1038/ncomms2628 OPEN CSN- and CAND1-dependent remodelling of the budding yeast SCF complex Aleksandra Zemla1, Yann Thomas1, Sylwia Kedziora1, Axel Knebel1, Nicola T Wood1, Gwenae¨l Rabut2 & Thimo Kurz1 Cullin–RING ligases (CRLs) are ubiquitin E3 enzymes with variable substrate-adaptor and -receptor subunits. All CRLs are activated by modification of the cullin subunit with the ubiquitin-like protein Nedd8 (neddylation). The protein CAND1 (Cullin-associated-Nedd8- dissociated-1) also promotes CRL activity, even though it only interacts with inactive ligase complexes. The molecular mechanism underlying this behaviour remains largely unclear. Here, we find that yeast SCF (Skp1–Cdc53–F-box) Cullin–RING complexes are remodelled in a CAND1-dependent manner, when cells are switched from growth in fermentable to non-fermentable carbon sources. Mechanistically, CAND1 promotes substrate adaptor release following SCF deneddylation by the COP9 signalosome (CSN). CSN- or CAND1- mutant cells fail to release substrate adaptors. This delays the formation of new complexes during SCF reactivation and results in substrate degradation defects. Our results shed light on how CAND1 regulates CRL activity and demonstrate that the cullin neddylation– deneddylation cycle is not only required to activate CRLs, but also to regulate substrate specificity through dynamic substrate adaptor exchange. 1 Scottish Institute for Cell Signalling, Protein Ubiquitylation Unit, College of Life Sciences, University of Dundee, Dow Street, Dundee DD1 5EH, Scotland, UK. 2 CNRS, Universite´ Rennes 1, Institut de Ge´ne´tique et De´veloppement de Rennes, 2 avenue du Professeur Le´on Bernard, CS 34317, Rennes Cedex 35043, France. -

CAND1-Dependent Control of Cullin 1-RING Ub Ligases Is Essential for Adipogenesis
Biochimica et Biophysica Acta 1833 (2013) 1078–1084 Contents lists available at SciVerse ScienceDirect Biochimica et Biophysica Acta journal homepage: www.elsevier.com/locate/bbamcr CAND1-dependent control of cullin 1-RING Ub ligases is essential for adipogenesis Dawadschargal Dubiel a,⁎, Maria Elka Gierisch b, Xiaohua Huang b, Wolfgang Dubiel b, Michael Naumann a a Institute of Experimental Internal Medicine, Medical Faculty, Otto von Guericke University, Leipziger Str. 44, 39120 Magdeburg, Germany b Department of General, Visceral, Vascular and Thoracic Surgery, Division of Molecular Biology, Charité, Universitätsmedizin Berlin, Charitéplatz 1, 10117 Berlin, Germany article info abstract Article history: Cullin-RING ubiquitin (Ub) ligases (CRLs) are responsible for ubiquitinylation of approximately 20% of all Received 14 September 2012 proteins degraded by the Ub proteasome system (UPS). CRLs are regulated by the COP9 signalosome (CSN) Received in revised form 20 December 2012 and by Cullin-associated Nedd8-dissociated protein 1 (CAND1). The CSN is responsible for removal of Accepted 7 January 2013 Nedd8 from cullins inactivating CRLs. CAND1 modulates the assembly of F-box proteins into cullin 1–RING Available online 14 January 2013 Ub ligases (CRL1s). We show that CAND1 preferentially blocks the integration of Skp2 into CRL1s. Suppres- sion of CAND1 expression in HeLa cells leads to an increase of the Skp2 assembly into CRL1s and to significant Keywords: COP9 signalosome reduction of the cyclin-dependent kinase (CDK) inhibitor p27. In contrary, CAND1 overexpression causes F-box proteins elevation of p27. The observed CAND1-dependent effects and CAND1 expression are independent of the Preadipocytes CSN as demonstrated in CSN1 knockdown cells. -

DDB1 Functions As a Linker to Recruit Receptor WD40 Proteins to CUL4− ROC1 Ubiquitin Ligases
Downloaded from genesdev.cshlp.org on October 6, 2021 - Published by Cold Spring Harbor Laboratory Press RESEARCH COMMUNICATION domain to bind with a conserved protein motif, the F DDB1 functions as a linker box, which, via its additional protein–protein interaction to recruit receptor WD40 modules, recruits various substrates, often phosphory- lated, to the CUL1–ROC1 catalytic core. To bring spe- proteins to CUL4–ROC1 cific substrates to CUL2- and CUL5-dependent ligases, a ubiquitin ligases heterodimeric linker complex containing elongins B and C binds simultaneously to an analogous N-terminal do- Yizhou Joseph He, Chad M. McCall, Jian Hu, main in CUL2 and CUL5 and to two similar protein 1 motifs, the VHL box and SOCS box. VHL and SOCS pro- Yaxue Zeng, and Yue Xiong teins, via their additional protein–protein interaction Lineberger Comprehensive Cancer Center, Department of modules, target various substrates differentially to the Biochemistry and Biophysics, Program in Molecular Biology CUL2–ROC1 or CUL5–ROC2 catalytic cores (Kamura et and Biotechnology, University of North Carolina, Chapel Hill, al. 1998, 2001, 2004; Stebbins et al. 1999; Zhang et al. North Carolina 27599, USA 1999). Omitting a linker, CUL3 utilizes its N-terminal domain to bind to proteins with a conserved 100-residue Cullins assemble the largest family of ubiquitin ligases protein motif known as a BTB domain, which, via addi- by binding with ROC1 and various substrate receptors. tional protein–protein interaction domains, then target CUL4 function is linked with many cellular processes, various substrates to the CUL3–ROC1 catalytic core (Fu- rukawa et al. 2003; Geyer et al. -

Double Minute Chromosomes in Glioblastoma Multiforme Are Revealed by Precise Reconstruction of Oncogenic Amplicons
Published OnlineFirst August 12, 2013; DOI: 10.1158/0008-5472.CAN-13-0186 Cancer Tumor and Stem Cell Biology Research Double Minute Chromosomes in Glioblastoma Multiforme Are Revealed by Precise Reconstruction of Oncogenic Amplicons J. Zachary Sanborn1,2,Sofie R. Salama2,3, Mia Grifford2, Cameron W. Brennan4, Tom Mikkelsen6, Suresh Jhanwar5, Sol Katzman2, Lynda Chin7, and David Haussler2,3 Abstract DNA sequencing offers a powerful tool in oncology based on the precise definition of structural rearrange- ments and copy number in tumor genomes. Here, we describe the development of methods to compute copy number and detect structural variants to locally reconstruct highly rearranged regions of the tumor genome with high precision from standard, short-read, paired-end sequencing datasets. We find that circular assemblies are the most parsimonious explanation for a set of highly amplified tumor regions in a subset of glioblastoma multiforme samples sequenced by The Cancer Genome Atlas (TCGA) consortium, revealing evidence for double minute chromosomes in these tumors. Further, we find that some samples harbor multiple circular amplicons and, in some cases, further rearrangements occurred after the initial amplicon-generating event. Fluorescence in situ hybridization analysis offered an initial confirmation of the presence of double minute chromosomes. Gene content in these assemblies helps identify likely driver oncogenes for these amplicons. RNA-seq data available for one double minute chromosome offered additional support for our local tumor genome assemblies, and identified the birth of a novel exon made possible through rearranged sequences present in the double minute chromosomes. Our method was also useful for analysis of a larger set of glioblastoma multiforme tumors for which exome sequencing data are available, finding evidence for oncogenic double minute chromosomes in more than 20% of clinical specimens examined, a frequency consistent with previous estimates. -

Partial Least Squares Based Gene Expression Analysis in Renal Failure Shuang Ding, Yinhai Xu, Tingting Hao and Ping Ma*
Ding et al. Diagnostic Pathology 2014, 9:137 http://www.diagnosticpathology.org/content/9/1/137 RESEARCH Open Access Partial least squares based gene expression analysis in renal failure Shuang Ding, Yinhai Xu, Tingting Hao and Ping Ma* Abstract Background: Preventive and therapeutic options for renal failure are still limited. Gene expression profile analysis is powerful in the identification of biological differences between end stage renal failure patients and healthy controls. Previous studies mainly used variance/regression analysis without considering various biological, environmental factors. The purpose of this study is to investigate the gene expression difference between end stage renal failure patients and healthy controls with partial least squares (PLS) based analysis. Methods: With gene expression data from the Gene Expression Omnibus database, we performed PLS analysis to identify differentially expressed genes. Enrichment and network analyses were also carried out to capture the molecular signatures of renal failure. Results: We acquired 573 differentially expressed genes. Pathway and Gene Ontology items enrichment analysis revealed over-representation of dysregulated genes in various biological processes. Network analysis identified seven hub genes with degrees higher than 10, including CAND1, CDK2, TP53, SMURF1, YWHAE, SRSF1,andRELA.Proteins encoded by CDK2, TP53,andRELA have been associated with the progression of renal failure in previous studies. Conclusions: Our findings shed light on expression character of renal failure patients with the hope to offer potential targets for future therapeutic studies. Virtual Slides: The virtual slide(s) for this article can be found here: http://www.diagnosticpathology.diagnomx.eu/vs/ 1450799302127207 Keywords: Renal failure, Partial least squares, Gene expression, Network Background detect dysregulated genes. -

H1 Linker Histones Silence Repetitive Elements by Promoting Both Histone H3K9 Methylation and Chromatin Compaction
H1 linker histones silence repetitive elements by promoting both histone H3K9 methylation and chromatin compaction Sean E. Healtona,1,2, Hugo D. Pintoa,1, Laxmi N. Mishraa, Gregory A. Hamiltona,b, Justin C. Wheata, Kalina Swist-Rosowskac, Nicholas Shukeirc, Yali Doud, Ulrich Steidla, Thomas Jenuweinc, Matthew J. Gamblea,b, and Arthur I. Skoultchia,2 aDepartment of Cell Biology, Albert Einstein College of Medicine, Bronx, NY 10461; bDepartment of Molecular Pharmacology, Albert Einstein College of Medicine, Bronx, NY 10461; cMax Planck Institute of Immunobiology and Epigenetics, Stübeweg 51, Freiburg D-79108, Germany; and dDepartment of Pathology, University of Michigan, Ann Arbor, MI 48109 Edited by Robert G. Roeder, Rockefeller University, New York, NY, and approved May 1, 2020 (received for review December 15, 2019) Nearly 50% of mouse and human genomes are composed of repetitive mechanisms of this regulation have not been fully explored. To sequences. Transcription of these sequences is tightly controlled during further investigate the roles of H1 in epigenetic regulation, we have development to prevent genomic instability, inappropriate gene used CRISPR-Cas9–mediated genome editing to inactivate addi- activation and other maladaptive processes. Here, we demonstrate tional H1 genes in the H1 TKO ES cells and thereby deplete the H1 an integral role for H1 linker histones in silencing repetitive elements in content to even lower levels. mouse embryonic stem cells. Strong H1 depletion causes a profound Nearly 50% of the mouse and human genomes consist of re- de-repression of several classes of repetitive sequences, including major petitive sequences, including tandem repeats, such as satellite se- satellite, LINE-1, and ERV. -

A Bacterial Protein Targets the BAHD1 Chromatin Complex to Stimulate Type III Interferon Response
A bacterial protein targets the BAHD1 chromatin complex to stimulate type III interferon response Alice Lebreton, Goran Lakisic, Viviana Job, Lauriane Fritsch, To Nam Tham, Ana Camejo, Pierre-Jean Matteï, Béatrice Regnault, Marie-Anne Nahori, Didier Cabanes, et al. To cite this version: Alice Lebreton, Goran Lakisic, Viviana Job, Lauriane Fritsch, To Nam Tham, et al.. A bacterial protein targets the BAHD1 chromatin complex to stimulate type III interferon response. Science, American Association for the Advancement of Science, 2011, 331 (6022), pp.1319-21. 10.1126/sci- ence.1200120. cea-00819299 HAL Id: cea-00819299 https://hal-cea.archives-ouvertes.fr/cea-00819299 Submitted on 26 Jul 2020 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Lebreton et al. Science 2011 doi:10.1126/science.1200120 A Bacterial Protein Targets the BAHD1 Chromatin Complex to Stimulate Type III Interferon Response Alice Lebreton1,2,3, Goran Lakisic4, Viviana Job5, Lauriane Fritsch6, To Nam Tham1,2,3, Ana Camejo7, Pierre-Jean Matteï5, Béatrice Regnault8, Marie-Anne Nahori1,2,3, Didier Cabanes7, Alexis Gautreau4, Slimane Ait-Si-Ali6, Andréa Dessen5, Pascale Cossart1,2,3* and Hélène Bierne1,2,3* 1.