H1 Linker Histones Silence Repetitive Elements by Promoting Both Histone H3K9 Methylation and Chromatin Compaction
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

How Human H1 Histone Recognizes DNA
molecules Article How Human H1 Histone Recognizes DNA Olesya P. Luzhetskaya, Sergey E. Sedykh and Georgy A. Nevinsky * Institute of Chemical Biology and Fundamental Medicine, SD of Russian Academy of Sciences, 8 Lavrentiev Ave., 630090 Novosibirsk, Russia; [email protected] (O.P.L.); [email protected] (S.E.S.) * Correspondence: [email protected]; Tel.: +7-383-363-51-26; Fax: +7-383-363-51-53 Received: 11 August 2020; Accepted: 1 October 2020; Published: 5 October 2020 Abstract: Linker H1 histone is one of the five main histone proteins (H1, H2A, H2B, H3, and H4), which are components of chromatin in eukaryotic cells. Here we have analyzed the patterns of DNA recognition by free H1 histone using a stepwise increase of the ligand complexity method; the affinity of H1 histone for various single- and double-stranded oligonucleotides (d(pN)n; n = 1–20) was evaluated using their competition with 12-mer [32P]labeled oligonucleotide and protein–oligonucleotide complex delaying on nitrocellulose membrane filters. It was shown that minimal ligands of H1 histone (like other DNA-dependent proteins and enzymes) are different mononucleotides (dNMPs; Kd = (1.30 0.2) 2 ± 10 M). An increase in the length of single-stranded (ss) homo- and hetero-oligonucleotides (d(pA)n, × − d(pT)n, d(pC)n, and d(pN)n with different bases) by one nucleotide link regardless of their bases, leads to a monotonic increase in their affinity by a factor of f = 3.0 0.2. This factor f corresponds ± to the Kd value = 1/f characterizing the affinity of one nucleotide of different ss d(pN)n for H1 at n = 2–6 (which are covered by this protein globule) is approximately 0.33 0.02 M. -
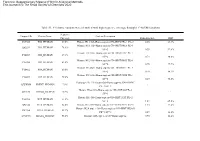
Table S1. 49 Histone Variants Were Identified with High Sequence Coverage Through LC-MS/MS Analysis Electronic Supplementary
Electronic Supplementary Material (ESI) for Analytical Methods. This journal is © The Royal Society of Chemistry 2020 Table S1. 49 histone variants were identified with high sequence coverage through LC-MS/MS analysis Sequence Uniprot IDs Protein Name Protein Description Coverage Ratio E2+/E2- RSD P07305 H10_HUMAN 67.5% Histone H1.0 OS=Homo sapiens GN=H1F0 PE=1 SV=3 4.85 23.3% Histone H1.1 OS=Homo sapiens GN=HIST1H1A PE=1 Q02539 H11_HUMAN 74.4% SV=3 0.35 92.6% Histone H1.2 OS=Homo sapiens GN=HIST1H1C PE=1 P16403 H12_HUMAN 67.1% SV=2 0.73 80.6% Histone H1.3 OS=Homo sapiens GN=HIST1H1D PE=1 P16402 H13_HUMAN 63.8% SV=2 0.75 77.7% Histone H1.4 OS=Homo sapiens GN=HIST1H1E PE=1 P10412 H14_HUMAN 69.0% SV=2 0.70 80.3% Histone H1.5 OS=Homo sapiens GN=HIST1H1B PE=1 P16401 H15_HUMAN 79.6% SV=3 0.29 98.3% Testis-specific H1 histone OS=Homo sapiens GN=H1FNT Q75WM6 H1FNT_HUMAN 7.8% \ \ PE=2 SV=3 Histone H1oo OS=Homo sapiens GN=H1FOO PE=2 Q8IZA3 H1FOO_HUMAN 5.2% \ \ SV=1 Histone H1t OS=Homo sapiens GN=HIST1H1T PE=2 P22492 H1T_HUMAN 31.4% SV=4 1.42 65.0% Q92522 H1X_HUMAN 82.6% Histone H1x OS=Homo sapiens GN=H1FX PE=1 SV=1 1.15 33.2% Histone H2A type 1 OS=Homo sapiens GN=HIST1H2AG P0C0S8 H2A1_HUMAN 99.2% PE=1 SV=2 0.57 26.8% Q96QV6 H2A1A_HUMAN 58.0% Histone H2A type 1-A OS=Homo sapiens 0.90 11.2% GN=HIST1H2AA PE=1 SV=3 Histone H2A type 1-B/E OS=Homo sapiens P04908 H2A1B_HUMAN 99.2% GN=HIST1H2AB PE=1 SV=2 0.92 30.2% Histone H2A type 1-C OS=Homo sapiens Q93077 H2A1C_HUMAN 100.0% GN=HIST1H2AC PE=1 SV=3 0.76 27.6% Histone H2A type 1-D OS=Homo sapiens P20671 -

UNIVERSITY of CALIFORNIA, SAN DIEGO Functional Analysis of Sall4
UNIVERSITY OF CALIFORNIA, SAN DIEGO Functional analysis of Sall4 in modulating embryonic stem cell fate A dissertation submitted in partial satisfaction of the requirements for the degree Doctor of Philosophy in Molecular Pathology by Pei Jen A. Lee Committee in charge: Professor Steven Briggs, Chair Professor Geoff Rosenfeld, Co-Chair Professor Alexander Hoffmann Professor Randall Johnson Professor Mark Mercola 2009 Copyright Pei Jen A. Lee, 2009 All rights reserved. The dissertation of Pei Jen A. Lee is approved, and it is acceptable in quality and form for publication on microfilm and electronically: ______________________________________________________________ ______________________________________________________________ ______________________________________________________________ ______________________________________________________________ Co-Chair ______________________________________________________________ Chair University of California, San Diego 2009 iii Dedicated to my parents, my brother ,and my husband for their love and support iv Table of Contents Signature Page……………………………………………………………………….…iii Dedication…...…………………………………………………………………………..iv Table of Contents……………………………………………………………………….v List of Figures…………………………………………………………………………...vi List of Tables………………………………………………….………………………...ix Curriculum vitae…………………………………………………………………………x Acknowledgement………………………………………………….……….……..…...xi Abstract………………………………………………………………..…………….....xiii Chapter 1 Introduction ..…………………………………………………………………………….1 Chapter 2 Materials and Methods……………………………………………………………..…12 -

Transcriptome Analyses of Rhesus Monkey Pre-Implantation Embryos Reveal A
Downloaded from genome.cshlp.org on September 23, 2021 - Published by Cold Spring Harbor Laboratory Press Transcriptome analyses of rhesus monkey pre-implantation embryos reveal a reduced capacity for DNA double strand break (DSB) repair in primate oocytes and early embryos Xinyi Wang 1,3,4,5*, Denghui Liu 2,4*, Dajian He 1,3,4,5, Shengbao Suo 2,4, Xian Xia 2,4, Xiechao He1,3,6, Jing-Dong J. Han2#, Ping Zheng1,3,6# Running title: reduced DNA DSB repair in monkey early embryos Affiliations: 1 State Key Laboratory of Genetic Resources and Evolution, Kunming Institute of Zoology, Chinese Academy of Sciences, Kunming, Yunnan 650223, China 2 Key Laboratory of Computational Biology, CAS Center for Excellence in Molecular Cell Science, Collaborative Innovation Center for Genetics and Developmental Biology, Chinese Academy of Sciences-Max Planck Partner Institute for Computational Biology, Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences, Shanghai 200031, China 3 Yunnan Key Laboratory of Animal Reproduction, Kunming Institute of Zoology, Chinese Academy of Sciences, Kunming, Yunnan 650223, China 4 University of Chinese Academy of Sciences, Beijing, China 5 Kunming College of Life Science, University of Chinese Academy of Sciences, Kunming, Yunnan 650204, China 6 Primate Research Center, Kunming Institute of Zoology, Chinese Academy of Sciences, Kunming, 650223, China * Xinyi Wang and Denghui Liu contributed equally to this work 1 Downloaded from genome.cshlp.org on September 23, 2021 - Published by Cold Spring Harbor Laboratory Press # Correspondence: Jing-Dong J. Han, Email: [email protected]; Ping Zheng, Email: [email protected] Key words: rhesus monkey, pre-implantation embryo, DNA damage 2 Downloaded from genome.cshlp.org on September 23, 2021 - Published by Cold Spring Harbor Laboratory Press ABSTRACT Pre-implantation embryogenesis encompasses several critical events including genome reprogramming, zygotic genome activation (ZGA) and cell fate commitment. -

Histone H1 Is Essential for Mitotic Chromosome Architecture
Published Online: 20 June, 2005 | Supp Info: http://doi.org/10.1083/jcb.200503031 JCB: ARTICLE Downloaded from jcb.rupress.org on May 12, 2019 Histone H1 is essential for mitotic chromosome architecture and segregation in Xenopus laevis egg extracts Thomas J. Maresca, Benjamin S. Freedman, and Rebecca Heald Department of Molecular and Cell Biology, University of California, Berkeley, Berkeley, CA 94720 uring cell division, condensation and resolution of spindle. Although functional kinetochores assembled, chromosome arms and the assembly of a func- aligned, and exhibited poleward movement, long and D tional kinetochore at the centromere of each sister tangled chromosome arms could not be segregated in chromatid are essential steps for accurate segregation of anaphase. Histone H1 depletion did not significantly affect the genome by the mitotic spindle, yet the contribution of the recruitment of known structural or functional chromo- individual chromatin proteins to these processes is poorly somal components such as condensins or chromokinesins, understood. We have investigated the role of embryonic suggesting that the loss of H1 affects chromosome archi- linker histone H1 during mitosis in Xenopus laevis egg tecture directly. Thus, our results indicate that linker histone extracts. Immunodepletion of histone H1 caused the assem- H1 plays an important role in the structure and function of bly of aberrant elongated chromosomes that extended off vertebrate chromosomes in mitosis. the metaphase plate and outside the perimeter of the Introduction Correct transmission of the genome by the mitotic spindle complexes condensin and cohesin, which are thought to form during cell division requires dramatic changes in chromosome ring structures that generate chromosome super coiling or architecture. -

Snf2h-Mediated Chromatin Organization and Histone H1 Dynamics Govern Cerebellar Morphogenesis and Neural Maturation
ARTICLE Received 12 Feb 2014 | Accepted 15 May 2014 | Published 20 Jun 2014 DOI: 10.1038/ncomms5181 OPEN Snf2h-mediated chromatin organization and histone H1 dynamics govern cerebellar morphogenesis and neural maturation Matı´as Alvarez-Saavedra1,2, Yves De Repentigny1, Pamela S. Lagali1, Edupuganti V.S. Raghu Ram3, Keqin Yan1, Emile Hashem1,2, Danton Ivanochko1,4, Michael S. Huh1, Doo Yang4,5, Alan J. Mears6, Matthew A.M. Todd1,4, Chelsea P. Corcoran1, Erin A. Bassett4, Nicholas J.A. Tokarew4, Juraj Kokavec7, Romit Majumder8, Ilya Ioshikhes4,5, Valerie A. Wallace4,6, Rashmi Kothary1,2, Eran Meshorer3, Tomas Stopka7, Arthur I. Skoultchi8 & David J. Picketts1,2,4 Chromatin compaction mediates progenitor to post-mitotic cell transitions and modulates gene expression programs, yet the mechanisms are poorly defined. Snf2h and Snf2l are ATP-dependent chromatin remodelling proteins that assemble, reposition and space nucleosomes, and are robustly expressed in the brain. Here we show that mice conditionally inactivated for Snf2h in neural progenitors have reduced levels of histone H1 and H2A variants that compromise chromatin fluidity and transcriptional programs within the developing cerebellum. Disorganized chromatin limits Purkinje and granule neuron progenitor expansion, resulting in abnormal post-natal foliation, while deregulated transcriptional programs contribute to altered neural maturation, motor dysfunction and death. However, mice survive to young adulthood, in part from Snf2l compensation that restores Engrailed-1 expression. Similarly, Purkinje-specific Snf2h ablation affects chromatin ultrastructure and dendritic arborization, but alters cognitive skills rather than motor control. Our studies reveal that Snf2h controls chromatin organization and histone H1 dynamics for the establishment of gene expression programs underlying cerebellar morphogenesis and neural maturation. -

Transcriptional Regulation by Histone Ubiquitination and Deubiquitination
Downloaded from genesdev.cshlp.org on September 30, 2021 - Published by Cold Spring Harbor Laboratory Press PERSPECTIVE Transcriptional regulation by histone ubiquitination and deubiquitination Yi Zhang1 Department of Biochemistry and Biophysics, Lineberger Comprehensive Cancer Center, University of North Carolina at Chapel Hill, North Carolina 27599, USA Ubiquitin (Ub) is a 76-amino acid protein that is ubiqui- The fact that histone ubiquitination occurs in the largely tously distributed and highly conserved throughout eu- monoubiquitinated form and is not linked to degrada- karyotic organisms. Whereas the extreme C-terminal tion, in combination with the lack of information regard- four amino acids are in a random coil, its N-terminal 72 ing the responsible enzymes, prevented us from under- amino acids have a tightly folded globular structure (Vi- standing the functional significance of this modification. jay-Kumar et al. 1987; Fig. 1A). Since its discovery ∼28 Recent identification of the E2 and E3 proteins involved years ago (Goldknopf et al. 1975), a variety of cellular in H2B ubiquitination (Robzyk et al. 2000; Hwang et al. processes including protein degradation, stress response, 2003; Wood et al. 2003a) and the discovery of cross-talk cell-cycle regulation, protein trafficking, endocytosis sig- between histone methylation and ubiquitination (Dover naling, and transcriptional regulation have been linked et al. 2002; Sun and Allis 2002) have set the stage for to this molecule (Pickart 2001). Ubiquitylation is pro- functional analysis of histone ubiquitination. In a timely posed to serve as a signaling module, and the informa- paper published in the previous issue of Genes & Devel- tion transmitted by this tag may depend on the nature of opment, Shelley Berger and colleagues (Henry et al. -

A Cytoplasmic Histone H1-Like Protein 2883 Ice-Cold RFB Buffer (160 Mm Kcl, 40 Mm Nacl, 20 Mm Na2egta, According to the Manufacturer’S Instruction
Research Article 2881 A novel linker histone-like protein is associated with cytoplasmic filaments in Caenorhabditis elegans Monika A. Jedrusik1, Stefan Vogt2, Peter Claus3 and Ekkehard Schulze1,* 1Georg-August University of Göttingen, Third Department of Zoology – Developmental Biology, Humboldtallee 34A, 37073 Göttingen, Germany 2Georg-August University of Göttingen, Institute for X-ray Physics, Geiststraße 11, 37073 Göttingen, Germany 3Hannover Medical School, Neuroanatomy, Carl-Neuberg-Str.1, 30625 Hannover, Germany *Author for correspondence (e-mail: [email protected]) Accepted 1 May 2002 Journal of Cell Science 115, 2881-2891 (2002) © The Company of Biologists Ltd Summary The histone H1 complement of Caenorhabditis elegans structure therein, the tonofilaments. Additionally contains a single unusual protein, H1.X. Although H1.X H1.X::GFP is expressed in the cytoplasm of body and vulva possesses the globular domain and the canonical three- muscle cells, neurons, excretory cells and in the nucleoli of domain structure of linker histones, the amino acid embryonic blastomeres and adult gut cells. RNA composition of H1.X is distinctly different from interference with H1.X results in uncoordinated and egg conventional linker histones in both terminal domains. We laying defective animals, as well as in a longitudinally have characterized H1.X in C. elegans by antibody labeling, enlarged pharynx. These phenotypes indicate a green fluorescent protein fusion protein expression and cytoplasmic role of H1.X in muscle growth and muscle RNA interference. Unlike normal linker histones, H1.X is function. a cytoplasmic as well as a nuclear protein and is not associated with chromosomes. H1.X is most prominently expressed in the marginal cells of the pharynx and is Key words: Caenorhabditis elegans, Histone H1, Chromatin, associated with a peculiar cytoplasmic cytoskeletal Intermediate filaments, Linker histone Introduction essential aspects of higher multicellular life, such as cell Linker histones are highly abundant eukaryotic chromatin differentiation and development. -

Anti-HIST1H1E K51ac Antibody
FOR RESEARCH USE ONLY! 02/20 Anti-HIST1H1E K51ac Antibody CATALOG NO.: A2051-100 (100 µl) BACKGROUND DESCRIPTION: Histones are basic nuclear proteins responsible for nucleosome structure of the chromosomal fiber in eukaryotes. Two molecules of each of the four core histones (H2A, H2B, H3, and H4) form an octamer, around which approximately 146 bp of DNA is wrapped in repeating units, called nucleosomes. The linker histone, H1, interacts with linker DNA between nucleosomes and functions in the compaction of chromatin into higher order structures. This gene is intronless and encodes a replication-dependent histone that is a member of the histone H1 family. Transcripts from this gene lack poly A tails but instead contain a palindromic termination element. This gene is found in the large histone gene cluster on chromosome 6. Histone H1.4 (Histone H1b) (Histone H1s-4), HIST1H1E, H1F4 ALTERNATE NAMES: ANTIBODY TYPE: Polyclonal HOST/ISOTYPE: Rabbit / IgG IMMUNOGEN: Acetylated peptide sequence targeting residues around Lysine 51 of human Histone H1.4 PURIFICATION: Antigen Affinity purified FORM: Liquid FORMULATION: In 0.01 M PBS, pH 7.4, 50% Glycerol, 0.03% proclin 300 SPECIES REACTIVITY: Human STORAGE CONDITIONS: Store at -20ºC. Avoid freeze / thaw cycles. APPLICATIONS AND USAGE: ICC 1:20-1:200, IF 1:50-1:200 This information is only intended as a guide. The optimal dilutions must be determined by the user Immunocytochemistry analysis of HeLa cells using Anti-HIST1H1E K51ac antibody at dilution of 1:100. Immunofluorescent analysis of HeLa cells (treated with sodium butyrate, 30 mM, 4 hrs) using Anti-HIST1H1E K51ac antibody at dilution of 1:100 and Alexa Fluor 488-conjugated Goat Anti-Rabbit IgG (H+L) as secondary antibody. -

Drosophila Ribosomal Proteins Are Associated with Linker Histone H1 and Suppress Gene Transcription
Downloaded from genesdev.cshlp.org on October 2, 2021 - Published by Cold Spring Harbor Laboratory Press Drosophila ribosomal proteins are associated with linker histone H1 and suppress gene transcription Jian-Quan Ni,1,3 Lu-Ping Liu,1,3 Daniel Hess,1 Jens Rietdorf,1 and Fang-Lin Sun1,2,4 1Friedrich Miescher Institute for Biomedical Research, Basel CH-4058, Switzerland; 2Institute of Epigenetics and Cancer Research, School of Medicine, Tsinghua University, Beijing 100080, China The dynamics and function of ribosomal proteins in the cell nucleus remain enigmatic. Here we provide evidence that specific components of Drosophila melanogaster ribosomes copurify with linker histone H1. Using various experimental approaches, we demonstrate that this association of nuclear ribosomal proteins with histone H1 is specific, and that colocalization occurs on condensed chromatin in vivo. Chromatin immunoprecipitation analysis confirmed that specific ribosomal proteins are associated with chromatin in a histone H1-dependent manner. Overexpression of either histone H1 or ribosomal protein L22 in Drosophila cells resulted in global suppression of the same set of genes, while depletion of H1 and L22 caused up-regulation of tested genes, suggesting that H1 and ribosomal proteins are essential for transcriptional gene repression. Overall, this study provides evidence for a previously undefined link between ribosomal proteins and chromatin, and suggests a role for this association in transcriptional regulation in higher eukaryotes. [Keywords: Ribosomal protein; L22; histone H1; chromatin; transcription] Supplemental material is available at http://www.genesdev.org. Received September 25, 2005; revised version accepted May 8, 2006. Transcription and translation in eukaryotes are generally discrete nuclear sites was sensitive to inhibitors of both believed to take place within two spatially separated cel- transcription and translation, arguing that the two pro- lular compartments. -

Deletions of IKZF1 and SPRED1 Are Associated with Poor Prognosis in A
Leukemia (2014) 28, 302–310 & 2014 Macmillan Publishers Limited All rights reserved 0887-6924/14 www.nature.com/leu ORIGINAL ARTICLE Deletions of IKZF1 and SPRED1 are associated with poor prognosis in a population-based series of pediatric B-cell precursor acute lymphoblastic leukemia diagnosed between 1992 and 2011 L Olsson1, A Castor2, M Behrendtz3, A Biloglav1, E Forestier4, K Paulsson1 and B Johansson1,5 Despite the favorable prognosis of childhood acute lymphoblastic leukemia (ALL), a substantial subset of patients relapses. As this occurs not only in the high risk but also in the standard/intermediate groups, the presently used risk stratification is suboptimal. The underlying mechanisms for treatment failure include the presence of genetic changes causing insensitivity to the therapy administered. To identify relapse-associated aberrations, we performed single-nucleotide polymorphism array analyses of 307 uniformly treated, consecutive pediatric ALL cases accrued during 1992–2011. Recurrent aberrations of 14 genes in patients who subsequently relapsed or had induction failure were detected. Of these, deletions/uniparental isodisomies of ADD3, ATP10A, EBF1, IKZF1, PAN3, RAG1, SPRED1 and TBL1XR1 were significantly more common in B-cell precursor ALL patients who relapsed compared with those remaining in complete remission. In univariate analyses, age (X10 years), white blood cell counts (4100 Â 109/l), t(9;22)(q34;q11), MLL rearrangements, near-haploidy and deletions of ATP10A, IKZF1, SPRED1 and the pseudoautosomal 1 regions on Xp/Yp were significantly associated with decreased 10-year event-free survival, with IKZF1 abnormalities being an independent risk factor in multivariate analysis irrespective of the risk group. Older age and deletions of IKZF1 and SPRED1 were also associated with poor overall survival. -

Gene Ontology Functional Annotations and Pleiotropy
Network based analysis of genetic disease associations Sarah Gilman Submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy under the Executive Committee of the Graduate School of Arts and Sciences COLUMBIA UNIVERSITY 2014 © 2013 Sarah Gilman All Rights Reserved ABSTRACT Network based analysis of genetic disease associations Sarah Gilman Despite extensive efforts and many promising early findings, genome-wide association studies have explained only a small fraction of the genetic factors contributing to common human diseases. There are many theories about where this “missing heritability” might lie, but increasingly the prevailing view is that common variants, the target of GWAS, are not solely responsible for susceptibility to common diseases and a substantial portion of human disease risk will be found among rare variants. Relatively new, such variants have not been subject to purifying selection, and therefore may be particularly pertinent for neuropsychiatric disorders and other diseases with greatly reduced fecundity. Recently, several researchers have made great progress towards uncovering the genetics behind autism and schizophrenia. By sequencing families, they have found hundreds of de novo variants occurring only in affected individuals, both large structural copy number variants and single nucleotide variants. Despite studying large cohorts there has been little recurrence among the genes implicated suggesting that many hundreds of genes may underlie these complex phenotypes. The question