New Records and Estimation of the Potential Distribution of the Stump-Tailed Porcupine Coendou Rufescens
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Chapter14.Pdf
PART I • Omora Park Long-Term Ornithological Research Program THE OMORA PARK LONG-TERM ORNITHOLOGICAL RESEARCH PROGRAM: 1 STUDY SITES AND METHODS RICARDO ROZZI, JAIME E. JIMÉNEZ, FRANCISCA MASSARDO, JUAN CARLOS TORRES-MURA, AND RAJAN RIJAL In January 2000, we initiated a Long-term Ornithological Research Program at Omora Ethnobotanical Park in the world's southernmost forests: the sub-Antarctic forests of the Cape Horn Biosphere Reserve. In this chapter, we first present some key climatic, geographical, and ecological attributes of the Magellanic sub-Antarctic ecoregion compared to subpolar regions of the Northern Hemisphere. We then describe the study sites at Omora Park and other locations on Navarino Island and in the Cape Horn Biosphere Reserve. Finally, we describe the methods, including censuses, and present data for each of the bird species caught in mist nets during the first eleven years (January 2000 to December 2010) of the Omora Park Long-Term Ornithological Research Program. THE MAGELLANIC SUB-ANTARCTIC ECOREGION The contrast between the southwestern end of South America and the subpolar zone of the Northern Hemisphere allows us to more clearly distinguish and appreciate the peculiarities of an ecoregion that until recently remained invisible to the world of science and also for the political administration of Chile. So much so, that this austral region lacked a proper name, and it was generally subsumed under the generic name of Patagonia. For this reason, to distinguish it from Patagonia and from sub-Arctic regions, in the early 2000s we coined the name “Magellanic sub-Antarctic ecoregion” (Rozzi 2002). The Magellanic sub-Antarctic ecoregion extends along the southwestern margin of South America between the Gulf of Penas (47ºS) and Horn Island (56ºS) (Figure 1). -

Full Day Visit to Cajas National Park/ Excursion Parque Nacional Cajas
Gran Colombia 11 – 02 & General Torres (2nd. Floor). Office 111. Building of “Asociación de Empleados” PH: +593 7 2837815 / +593 7 2837681 24 hours Mob: +593 999 604870 or +593 999 750193 Website: www.apullacta.com E-mail: [email protected] / [email protected] /[email protected] CUENCA – ECUADOR FULL DAY VISIT TO CAJAS NATIONAL PARK/ EXCURSION PARQUE NACIONAL CAJAS Cajas National Park offers us impressive landscapes of cloud forest and Andean moorland. The mysterious silence of its mountains invites us to wander the incredible paths bordering some of its lakes. There are more than 200 lakes and lagoons. During the walk we observe interesting endemic plants as well as birds native to the region. Enjoy contact with nature in this unique place in the Ecuadorian Andes. El Parque Cajas nos ofrece impresionantes paisajes de bosque nublado y paramo Andino. El misterioso silencio de sus montañas nos invita a recorrer sus increíbles senderos bordeando algunas lagunas. Existen más de 200 lagunas. Durante la caminata observamos interesantes plantas endémicas, así como aves propias del lugar. Disfruta del contacto con la naturaleza en este lugar único en los Andes Ecuatorianos. The Trip: Pick-up at the hotel Transfer from Cuenca to Cajas National Park, directly to the Llaviuco zone in the lower part at 3100 meters above sea level. The path is level and easy to navigate through the woods, taking between 45 minutes and an hour. Then we continue to the higher region of the Park called Tres Cruces (Three Crosses) at approximately 4160 meters above sea level. There we will have a panoramic view of the Park with time for taking photographs and listening to information from the guide. -

Página 1 De 8 ESCUELA SUPERIOR POLITÉCNICA DEL LITORAL
ESCUELA SUPERIOR POLITÉCNICA DEL LITORAL FACULTAD DE CIENCIAS NATURALES Y MATEMÁTICAS DEPARTAMENTO DE CIENCIAS QUÍMICAS Y AMBIENTALES Año Académico: 2017 – 2018 Semestre: II Materia: Introducción a la Gestión Profesor: Ambiental Evaluación: Tercera Fecha: 23 febrero 2018 COMPROMISO DE HONOR Yo, ………………………………………………………………………………………………………………..…………… al firmar este compromiso, reconozco que el presente examen está diseñado para ser resuelto de manera individual, que puedo usar una calculadora ordinaria para cálculos aritméticos, un lápiz o esferográfico; que solo puedo comunicarme con la persona responsable de la recepción del examen; y, cualquier instrumento de comunicación que hubiere traído, debo apagarlo y depositarlo en la parte anterior del aula, junto con algún otro material que se encuentre acompañándolo. No debo además, consultar libros, notas, ni apuntes adicionales a las que se entreguen en esta evaluación. Los temas debo desarrollarlos de manera ordenada. Firmo al pie del presente compromiso, como constancia de haber leído y aceptar la declaración anterior. "Como estudiante de ESPOL me comprometo a combatir la mediocridad y actuar con honestidad, por eso no copio ni dejo copiar". Firma NÚMERO DE MATRÍCULA:……………….…. PARALELO:………… TOPIC I. CRITICAL KNOWLEDGE ANALYSIS (20 points): In each item write the correct answer that corresponds to the blank space that is underlined in each item. 1. An organism that uses the process of photosynthesis to produce nutrients and organic matter is called 2. An ecosystem that owns animals and plants characteristic of that site and average levels of specific precipitation and temperature is called 3. To name a species considered as PRODUCTIONER at the trophic levels that could be found in the Polytechnic Campus (Protector Forest) 4. -

How to Cite Complete Issue More Information About This Article
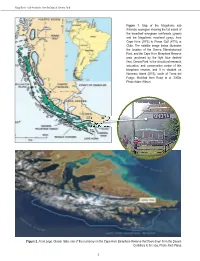
Therya ISSN: 2007-3364 Asociación Mexicana de Mastozoología A. C. Narváez Romero, Carlos; Reyes Puig, Carolina; Valle, Darwin; Brito, Jorge New records and estimation of the potential distribution of the Stump-Tailed Porcupine Coendou rufescens Therya, vol. 9, no. 2, 2018, May-June, pp. 137-146 Asociación Mexicana de Mastozoología A. C. DOI: https://doi.org/10.12933/therya-18-581 Available in: https://www.redalyc.org/articulo.oa?id=402358306005 How to cite Complete issue Scientific Information System Redalyc More information about this article Network of Scientific Journals from Latin America and the Caribbean, Spain and Journal's webpage in redalyc.org Portugal Project academic non-profit, developed under the open access initiative THERYA, 2018, Vol. 9 (2): 137-146 DOI: 10.12933/therya-18-581 ISSN 2007-3364 New records and estimation of the potential distribution of the Stump-Tailed Porcupine Coendou rufescens CARLOS NARVÁEZ-ROMERO1, CAROLINA REYES-PUIG2, DARWIN VALLE3, AND JORGE BRITO4,5* 1 Coordinación Región Austro, Programa para la Conservación de los Murciélagos del Ecuador (PCME), Asociación Ecuatoriana de Mastozoología, Quito. Ecuador. Email: [email protected] (CNR). 2 Instituto de Zoología Terrestre, Museo de Zoología, Instituto BIOSFERA, Colegio de Ciencias Biológicas y Ambientales, Universidad San Francisco de Quito (USFQ) campus Cumbayá, Casilla Postal 17-1200-841, Quito. Ecuador. Email: [email protected] (CRP) 3 Equanativa Cía. Ltda, Loja, Ecuador. Email: [email protected] (DV). 4 Departamento de Biología, Facultad de Ciencias, Escuela Politécnica Nacional, Casilla 17-01-2759, Quito, Ecuador. 5 Instituto Nacional de Biodiversidad, Calle Rumipamba 341 y Av. de los Shyris, Casilla: 17-07-8976, Quito. -

United Nations Development Programme Country: Peru PROJECT DOCUMENT1
United Nations Development Programme Country: Peru PROJECT DOCUMENT1 Project Title: Transforming Management of Protected Area/Landscape Complexes to Strengthen Ecosystem Resilience UNDAF Outcome(s): The State, with the participation of civil society, private sector, and academic and scientific institutions, will have designed, implemented and/or strengthened policies, programs, and plans, with a focus on environmental sustainability, for the sustainable management of natural resources and biodiversity conservation. UNDP Strategic Plan Primary Outcome: Growth is inclusive and sustainable, incorporating productive capacities that create employment and livelihoods for the poor and excluded. Expected CP Outcome(s): See UNDAF Outcome Expected CPAP Output(s): Management instruments designed and in early implementation that contribute to the conservation and sustainable use of biodiversity at the local, regional and national scales. Executing Entity/Implementing Partner: National Protected Areas Service (SERNANP) Implementing Entity/Responsible Partners: UNDP Brief Description This project will transform the management of vulnerable ecosystems in Peru to alleviate the direct and indirect impacts of climate change (CC) on globally significant biodiversity and ecosystem functionality, through a three-pronged approach: development of management systems (monitoring and early warning systems, management decision making tools and sustainable financing) in order to optimize national readiness to address the implications of CC on ecosystems; expanding and strengthening PAs in landscapes that are particularly sensitive to CC, in order to protect refugia and corridors and build readiness to address specific CC impacts; and promoting sustainable land management in landscapes surrounding PAs in order to anticipate increased threats from current land uses for BD and ecosystem functions. This will reduce pressures on ecosystems and make them more resilient to expected CC impacts. -

Interpretación De La Calidad Ambiental De Un Ecosistema Estratégico De Alta Montaña Y Su Influencia En La Salud Ambiental, Caso Estudio Páramo De Pisba
INTERPRETACIÓN DE LA CALIDAD AMBIENTAL DE UN ECOSISTEMA ESTRATÉGICO DE ALTA MONTAÑA Y SU INFLUENCIA EN LA SALUD AMBIENTAL, CASO ESTUDIO PÁRAMO DE PISBA Daniela Doncel Prada Mariana Andrea Roas Díaz Universidad El Bosque Facultad de Ingeniería Programa Ingeniería Ambiental Bogotá, 12 de Mayo de 2020 1 INTERPRETACIÓN DE LA CALIDAD AMBIENTAL DE UN ECOSISTEMA ESTRATÉGICO DE ALTA MONTAÑA Y SU INFLUENCIA EN LA SALUD AMBIENTAL, CASO ESTUDIO PÁRAMO DE PISBA Daniela Doncel Prada Mariana Andrea Rojas Díaz Trabajo de investigación presentado como requisito parcial para optar al título de: Ingeniero Ambiental Director (a): Claudia Patricia Gómez Rendón Línea de Investigación: Salud Ambiental Universidad El Bosque Facultad de Ingeniería Programa Ingeniería Ambiental Bogotá, Colombia Año 2020 2 Acta de sustentación 3 Nota de Salvedad de Responsabilidad Institucional La Universidad El Bosque, no se hace responsable de los conceptos emitidos por los investigadores en su trabajo, solo velará por el rigor científico, metodológico y ético del mismo en aras de la búsqueda de la verdad y la justicia. 4 Dedicatoria y agradecimientos Le damos gracias a Dios por permitirnos culminar satisfactoriamente este proyecto que nos enriqueció y fortaleció como personas, estudiantes y profesionales. A nuestras familias, en especial a nuestros padres y hermanos que con mucho amor nos acompañaron, motivaron, y apoyaron en el transcurso de la carrera y de la realización de este trabajo. A nuestra directora, la profe Claudia Gómez quien con mucha dedicación nos transmitió su conocimiento, nos orientó, enriqueció y acompañó de principio a fin en el desarrollo del proyecto. Al profesor Andrés, Julián Avella y los habitantes de la Vereda Pueblo Viejo por permitirnos vivir esa maravillosa experiencia en campo, a los niños de la escuela por enseñarnos tanto con tan poco, a Doña Histenia y Don Jesús por recibirnos con mucha amabilidad en su hogar y acogernos durante nuestra estadía en el Páramo de Pisba. -
A History of Water in 20Th-Century Bogotá
The City's Currents: A History of Water in 20th-Century Bogotá Stefania Gallini, Laura Felacio, Angélica Agredo, and Stephanie Garcés This exhibition explores the role of water in the struggle of Colombia’s capital to become a modern city. For Bogotá, complying with the standards of sanitary reformers and the governing elite was a social, technological, and environmental control endeavor. The exhibition examines everyday and gendered experiences of water, such as washing clothes or bathing, as well as urban infrastructural interventions including the domestication of rivers for water supply and wastewater disposal systems. This exhibition has been created by Stefania Gallini, Laura Felacio, Angélica Agredo and Stephanie Garcés (2014) under a CC BY-NC-SA 4.0 International license, this refers only to the text. For copyright information on the above images, please click here. http://www.environmentandsociety.org/exhibitions/water-bogota/copyright-information How to cite: Gallini, Stefania, Laura Felacio, Angélica Agredo, and Stephanie Garcés. “The City’s Currents: A History of Water in 20th-Century Bogotá.” Environment & Society Portal, Virtual Exhibitions 2014, no. 3. Rachel Carson Center for Environment and Society. doi.org/10.5282/rcc/6295 . ISSN 2198-7696 Environment & Society Portal, Virtual Exhibitions Source URL: http://www.environmentandsociety.org/node/6295 PDF created on: 11 March 2021 13:40:31 Introduction Paisaje del Río Bogotá (Colombia) S.A. The landscape of the Bogotá River. One of the riverbanks is planted entirely with eucalyptus. (Photograph by Gumersindo Cuéllar Jiménez. © Biblioteca Luis Ángel Arango.) © Biblioteca Luis Ángel Arango. Banco de la República de Colombia. Courtesy of Mario Cuéllar Bobadilla. -
Content Catalogue
CONTENT CATALOGUE Consejo Nacional de las Asamblea & Artes y la Cultura en Junta Directiva Cámara de Comercio Cinematografía - CNACC Proimágenes Colombia de Bogotá Junta Directiva MARIANA GARCÉS CÓRDOBA MARIANA GARCÉS CÓRDOBA MÓNICA DE GREIFF LINDO Ministra de Cultura Ministra de Cultura Presidencia Ejecutiva ADELFA MARTÍNEZ ENZO ARIZA GUILLERMO BOTERO NIETO Directora de Cinematografía Secretario General Presidente Junta Directiva DARÍO VARGAS LINARES ADELFA MARTÍNEZ WILLIAM PARRA DURÁN Delegado Ministra de Cultura Directora de Cinematografía Vicepresidente HÉCTOR ROSAS JUANITA RODRÍGUEZ REPRESENTANTES DEL Representante Distribuidores Ministerio de las Tecnologías SECTOR PRIVADO de la Información y las ANDRÉS SÁNCHEZ DUQUE Comunicaciones Principales: Representante Guillermo BOTERO NIETO Sector Artístico/Creativo YANETH GIHA TOVAR Gonzalo SERRANO RODRÍGUEZ Ministerio de Educación Juan Diego TRUJILLO MEJÍA CARLOS ENRIQUE Carlos Augusto RAMÍREZ OGLIASTRI GARCÉS JAVIER OLARTE TRIANA Jaime Alfonso Representante Exhibidores Universidad Nacional MANTILLA GARCÍA de Colombia Gonzalo ECHEVERRY GARZÓN SERGIO SÁNCHEZ ÁLVAREZ Germán Alfredo Representante Directores HERNANDO GALLO BERNAL ORTIZ CÁRDENAS DIAN Francisco DURÁN CASAS EDGAR MONTAÑEZ MUÑOZ Representante Sector Técnico GILBERTO GALLEGO Suplentes: Cine Colombia S.A. Jorge Arnulfo PACHÓN FERNANDO Juan David ÁNGEL BOTERO CHARRY GONZÁLEZ FERNÁN Hugo Fernando GARCÍA Representante GARCÍA DE LA TORRE Sandra NEIRA LIÉVANO Consejos Departamentales Asociación Colombiana de Luis Fernando ÁNGEL MORENO -

Assessing the Impact of Land Use Changes on Hydropower Production and Erosion in the Coca River Basin
ASSESSING THE IMPACT OF LAND USE CHANGES ON HYDROPOWER PRODUCTION AND EROSION IN THE COCA RIVER BASIN. A CONTRIBUTION TOWARDS INTEGRATED WATER RESOURCES MANAGEMENT IN ECUADOR. Zur Erlangung des akademischen Grades eines DOKTOR-INGENIEURS (Dr.-Ing.) von der KIT - Fakultät für Bauingenieur-, Geo- und Umweltwissenschaften des Karlsruher Instituts für Technologie (KIT) genehmigte DISSERTATION von Carlos Alberto Zuleta Salmon aus Ecuador Tag der mündlichen Prüfung: 18. Mai 2021 Referent: Dr.-Ing. Uwe Ehret Korreferent: Jun.-Prof. Dr.rer.nat. Andreas Braun Karlsruhe 2021 Contents List of Figures ....................................................................................................................................................... iii List of Tables ........................................................................................................................................................ iv Abstract .................................................................................................................................................................. v German Abstract ................................................................................................................................................. vii CHAPTER 1: Introduction .................................................................................................................................. 1 1.1- General overview ................................................................................................................................ -

Escuela Superior Politécnica De Chimborazo Facultad De Recursos Naturales Escuela De Ingeniería En Ecoturismo
ESCUELA SUPERIOR POLITÉCNICA DE CHIMBORAZO FACULTAD DE RECURSOS NATURALES ESCUELA DE INGENIERÍA EN ECOTURISMO DISEÑO DE MEDIOS INTERPRETATIVOS PARA LA RUTA INGUISAY – VOLCÁN EL ALTAR EN EL PARQUE NACIONAL SANGAY, PROVINCIA DE CHIMBORAZO TRABAJO DE TITULACIÓN PROYECTO TÉCNICO PARA TITULACIÓN DE GRADO PRESENTADA COMO REQUISITO PARCIAL PARA OBTENER EL TÍTULO DE INGENIERA EN ECOTURISMO GORDILLO COBO SILVIA CRISTINA RIOBAMBA- ECUADOR 2018 ii ©2018, Silvia Cristina Gordillo Cobo Se autoriza la producción total o parcial, con fines académicos, por cualquier medio o procedimiento, incluyendo la cita bibliográfica del documento, siempre y cuando se reconozca el Derecho de Autor. iii iv v vi DEDICATORIA Dedico este trabajo a mi familia la cual ha sido mi apoyo y fortaleza para crecer como persona, al igual que a mis profesores los cuales han sido mis guías en el transcurso de este camino. vii AGRADECIMIENTO Agradezco sobre todo a Dios por brindarme la oportunidad de haber culminado esta etapa en mis estudios, la cual es un peldaño más para seguir superándome como profesional y como ser humano. A mi madre por ser mi soporte incondicional en la vida. A mi hija que ha sido mi motivación para seguir adelante en todo este proceso. Al Ministerio del Ambiente por haberme abierto las puertas para la realización de mi trabajo de titulación A la Escuela Superior Politécnica de Chimborazo, sobre todo a la Escuela de Ingeniería en ecoturismo, que a través de sus profesores han sabido inculcarme valores y conocimientos que me ayudarán para desenvolverme adecuadamente en el ámbito profesional. viii TABLA DE CONTENIDO DISEÑO DE MEDIOS INTERPRETATIVOS PARA LA RUTA INGUISAY – VOLCÁN EL ALTAR EN EL PARQUE NACIONAL SANGAY, PROVINCIA DE CHIMBORAZO .... -
Mountain Forum Bulletin January 2008
Mountain Forum Bulletin January 2008 Climate Change in Mountains: Impact and Adaptation u Intangible Ecologies: Sacred Mountain Landscapes in a Changing Climate u Climate Change Impacts: Winter Tourism Adaptation Strategies u Effect of Climatic Change on the Endangered Dracaena Ombet Tree in the Gabel Elba Protected Area in Egypt u Experience of a Herder’s Life in Western Bhutan u Climate Change: Opportunity or Threat in the Central Andean Region of Peru http://www.mtnforum.org Mountain Forum Bulletin Volume VIII Issue 1, January 2008 ISSN 1029-3760 Editor: Marianne Heredge Publication Coordination Team: Ujol Sherchan, Suman Jaiswal, Shreeza Rajbhandari Design: Payas Chhetri Cover photographs (clockwise from right) Glacier at Gaumukh, Uttarakhand, India. Photo: G.Areendran Tribal settlements in Kurangani Hills (Mudhuvakkudi, Muttam hamlets). Photo: P. S. Bose Beech (Fagus sylvatica) tree line in the East Carpathians. Photo: V. Kricsfalusy Snow cover decrease and climate change, French Alps. Photo: Guillaume Prudent Published by the Mountain Forum Secretariat in cooperation with regional nodes in Africa, Asia-Pacific, Europe, Latin America and North America. The opinions expressed by contributors to the Mountain Forum Bulletin are not necessarily those of the Mountain Forum Secretariat, regional nodes of the Mountain Forum, or any of their host institutions. The Mountain Forum Secretariat reserves the right to edit contributions for the sake of clarity and brevity. Contents Editorial 2 Feature Intangible Ecologies: Sacred Mountain Landscapes -

Lynx Rufus) in the Potosino-Zacatecano Plateau, Mexico Raúl Sánchez-González, Anuar David Hernández-Saint Martin, Octavio Cesar Rosas- Rosas, Juan García-Chávez 107
www.mastozoologiamexicana.org La Portada Zorrillo espalda blanca (Conepatus leuconotus) es una de las ocho especies de zorrillos que se encentran en México. Su distribución incluye gran parte de Norte América, incluyendo mayormente a México. Conepatus leuconotus es una especie relativamente abundante y muy conspicua donde se encuentra, la especie es casada constantemente sin razón, aunque en algunos lugares de México consideran que su carne es medicinal. La foto fue tomada en las planicies del istmo de Tehuantepec (Fotografía de Sergio Ticul Álvarez Castañeda). Nuestro logo “Ozomatli” El nombre de “Ozomatli” proviene del náhuatl se refiere al símbolo astrológico del mono en el calendario azteca, así como al dios de la danza y del fuego. Se relaciona con la alegría, la danza, el canto, las habilidades. Al signo decimoprimero en la cosmogonía mexica. “Ozomatli” es una representación pictórica de los mono arañas (Ateles geoffroyi). La especie de primate de más amplia distribución en México. “ Es habitante de los bosques, sobre todo de los que están por donde sale el sol en Anáhuac. Tiene el dorso pequeño, es barrigudo y su cola, que a veces se enrosca, es larga. Sus manos y sus pies parecen de hombre; también sus uñas. Los Ozomatin gritan y silban y hacen visajes a la gente. Arrojan piedras y palos. Su cara es casi como la de una persona, pero tienen mucho pelo.” THERYA Volumen 9, número 2 mayo 2018 ARTICLES Diet and abundance of bobcat (Lynx rufus) in the Potosino-Zacatecano Plateau, Mexico Raúl Sánchez-González, Anuar David Hernández-Saint Martin, Octavio Cesar Rosas- Rosas, Juan García-Chávez 107 Annual cycle of the mexican ground squirrel (Xerospermophilus perotensis) Sandra H.