Recent Advances in Salivary Scintigraphic Evaluation of Salivary Gland Function
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Research Article Magnetic Resonance Sialography Findings of Submandibular Ducts Imaging
Hindawi Publishing Corporation BioMed Research International Volume 2013, Article ID 417052, 6 pages http://dx.doi.org/10.1155/2013/417052 Research Article Magnetic Resonance Sialography Findings of Submandibular Ducts Imaging Nezahat Karaca ErdoLan,1 Canan Altay,2 Nesibe Özenler,3 TuLba Bozkurt,1 Engin Uluç,1 Berna Dirim Mete,1 and Esmail Özdemir4 1 Department of Radiology, Izmir Ataturk¨ Research and Training Hospital, Basın Sitesi, Karabaglar,˘ 35360 Izmir, Turkey 2 Department of Radiology, Medical School, Dokuz Eylul University, Inciralti, 35340 Izmir, Turkey 3 Department of Radiology, Balıkesir Ataturk¨ State Hospital, Yıldız Mahallesi Soma Caddesi No. 1, 10100 Balıkesir, Turkey 4 Universal Ege Health Hospital, 35220 Izmir, Turkey Correspondence should be addressed to Canan Altay; [email protected] Received 2 April 2013; Revised 29 May 2013; Accepted 12 June 2013 Academic Editor: Yoshito Tsushima Copyright © 2013 Nezahat Karaca Erdogan˘ et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Purpose. We aimed to assess the problem solving capability of magnetic resonance sialography (MR sialography), a noninvasive method for imaging submandibular gland ducts and determining duct-related pathologies, by comparing diseased and healthy cases. Materials and Methods. We conducted radiological assessment on a total of 60 submandibular glands (mean age 44.7) in 20 cases and 10 volunteers. MR sialography examinations were conducted with single-shot fast spin-echo sequence by using a surface coil placed on the submandibular gland. Each gland was evaluated in terms of the length, width and stricture of the main duct, as well as the difference between the intraparenchymal duct width, and the main duct width. -
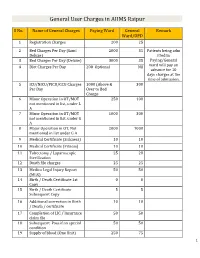
General User Charges in AIIMS Raipur
General User Charges in AIIMS Raipur S No. Name of General Charges Paying Ward General Remark Ward/OPD 1 Registration Charges 200 25 2 Bed Charges Per Day (Sami 2000 35 Patients being adm Deluxe) itted in 3 Bed Charges Per Day (Deluxe) 3000 35 Paying/General 4 Diet Charges Per Day 200 Optional Nil ward will pay an advance for 10 days charges at the time of admission. 5 ICU/NICU/PICU/CCU Charges 1000 (Above & 300 Per Day Over to Bed Charge 6 Minor Operation in OT/MOT 250 100 not mentioned in list, under L A 7 Minor Operation in OT/MOT 1000 300 not mentioned in list, under G A 8 Major Operation in OT, Not 2000 1000 mentioned in list under G A 9 Medical Certificate (Sickness) 10 10 10 Medical Certificate (Fitness) 10 10 11 Tubectomy / Laparoscopic 25 20 Sterilization 12 Death file charges 25 25 13 Medico Legal Injury Report 50 50 (MLR) 14 Birth / Death Certificate 1st 0 0 Copy 15 Birth / Death Certificate 5 5 Subsequent Copy 16 Additional correction in Birth 10 10 / Death / certificate 17 Completion of LIC / Insurance 50 50 claim file 18 Subsequent Pass if on special 50 50 condition 19 Supply of blood (One Unit) 250 75 1 20 Medical Board Certificate 500 500 On Special Case User Charges for Investigations in AIIMS Raipur S No. Name of Investigations Paying General Remark Ward Ward/OPD Anaesthsia 1 ABG 75 50 2 ABG ALONGWITH 150 100 ELECTROLYTES(NA+,K+)(Na,K) 3 ONLY ELECTROLYTES(Na+,K+,Cl,Ca+) 75 50 4 ONLY CALCIUM 50 25 5 GLUCOSE 25 20 6 LACTATE 25 20 7 UREA. -

Procedure Codes for Physician: Radiology
NEW YORK STATE MEDICAID PROGRAM PHYSICIAN - PROCEDURE CODES SECTION 4 - RADIOLOGY Physician – Procedure Codes, Section 4 - Radiology Table of Contents GENERAL INSTRUCTIONS ............................................................................................................ 4 GENERAL RULES AND INFORMATION ......................................................................................... 6 MMIS RADIOLOGY MODIFIERS .................................................................................................... 8 DIAGNOSTIC RADIOLOGY (DIAGNOSTIC IMAGING)................................................................. 9 HEAD AND NECK.................................................................................................................... 9 CHEST .................................................................................................................................. 10 SPINE AND PELVIS .............................................................................................................. 11 UPPER EXTREMITIES .......................................................................................................... 12 LOWER EXTREMITIES ......................................................................................................... 13 ABDOMEN ............................................................................................................................ 14 GASTROINTESTINAL TRACT ............................................................................................... 15 URINARY -

Use of MR Sialography and T2W Fat-Suppressed MR in Parotitis
TIPS FOR THE RADIOLOGIST Use of MR sialography and T2W fat-suppressed MR in parotitis Findings and diagnosis Phil Pretorius, FFRad (Diag) SA A young girl presented with only a non-specific history of recurrent Drs Visser, Vawda, Erasmus and Partners, Greenacres Hospital, Port painful swelling of both parotid glands over the preceding few months. Elizabeth An US scan showed numerous small, discrete hypoechoic foci throughout both parotid glands. An MRI was recommended and performed using standard sequences including thin-slice axial and coronal T1 and T2 (fat-suppressed) sequences. In view of the pathological changes seen Background on US, fat-suppressed T1 series were also obtained, as were pre- and post-contrast axial and coronal series. An MR sialogram was acquired Imaging can be helpful when investigating salivary gland pain and through each parotid gland in an oblique sagittal orientation, which swelling. Typically in such cases, an ultrasound (US) or conventional proved to be the most useful diagnostic sequence (Fig. 1). The images sialogram would be requested in seeking obstructed or dilated ducts, a from these sequences showed both parotid glands to be diffusely swollen ductal calculus or an abscess within the gland. Occasionally a computed with numerous microcysts within the gland parenchyma. This was felt tomography (CT) scan is requested. Magnetic resonance imaging (MRI) to be typical of Sjögrens disease, Stage I or II (depending on the size of is usually reserved for investigating clinically palpable masses in the the cysts). glands. In acute non-obstructive parotitis, the differential diagnosis includes Discussion acute non-suppurative parotitis (as found in mumps), early Sjögren’s Sjögrens syndrome is an auto-immune disease affecting the lacrimal syndrome, or suppurative parotitis as seen following duct obstruction. -

Role of Magnetic Resonance Sialography in Diagnosis of Salivary Gland Diseases M Elhalal, Y Khattab, E El-Sayed, R Habib
The Internet Journal of Radiology ISPUB.COM Volume 21 Number 1 Role of Magnetic Resonance Sialography in Diagnosis of Salivary Gland Diseases M Elhalal, Y Khattab, E El-Sayed, R Habib Citation M Elhalal, Y Khattab, E El-Sayed, R Habib. Role of Magnetic Resonance Sialography in Diagnosis of Salivary Gland Diseases. The Internet Journal of Radiology. 2018 Volume 21 Number 1. DOI: 10.5580/IJRA.53225 Abstract Objectives: Study the role of MR Sialography in diagnosis of salivary diseases and compare the findings with conventional sialography. Background: One of many modalities to evaluate the ductal system of major salivary glands is magnetic resonance sialography. It’s based on the intrinsic hyperintensity of static fluid on heavily T2-weighted images. Several studies have compared MR Sialography with conventional sialography and demonstrated that MR Sialography is generally as accurate as conventional sialography. Additionally, MR Sialography is a noninvasive technique that doesn’t require the cannulation and injection of contrast medium and doesn’t use ionizing radiation Patients and methods: The study was conducted in Misr Radiology Center on 27 patients with symptoms like swelling, pain and/or feeling of discharge from duct orifice. The study is performed between October 2012 and December 2014. Results: Out of the 27 patients studied (17 male and 10 female), 24 have done both conventional and MR Sialography. Their ages ranged from 22 to 69 years. In comparison with conventional sialography, the MR Sialography revealed accuracy of 79% in showing the main duct, 92% as regarding sialolithiasis, 100% as regarding strictures, 100% as regarding sialectasis and a total accuracy of 92% in evaluation of the ductal system of the major salivary glands. -

Scintigraphic Assessment of Salivary Gland Function in a Rat Model
in vivo 24: 681-686 (2010) Scintigraphic Assessment of Salivary Gland Function in a Rat Model ANDREAS PFESTROFF1, FELIX MÜLLER2, DAMIANO LIBRIZZI1, BEHFAR EIVAZI2, MARTIN BEHE3, HELMUT HOEFFKEN1, THOMAS M. BEHR1 and AFSHIN TEYMOORTASH2 Departments of 1Nuclear Medicine and 2Otolaryngology, Head and Neck Surgery, Philipps University, 35043 Marburg, Germany; 3Division of Nuclear Medicine and PET Center, University Hospital of Freiburg, 79106 Freiburg, Germany Abstract. Background: Salivary gland dysfunction with examinations (6, 28). However, the debate on the most reliable xerostomia is a major clinical problem without a causal and suitable parameters for the diagnosis of loss of salivary therapy in most cases. The development of an animal model gland function persists (29). Direct evaluation of salivary for scintigraphic assessment of salivary gland function has gland function may be performed by analysis of saliva after great clinical relevance for the investigation of promising its collection (17, 19). The saliva can be collected directly new diagnostic and therapeutic strategies for chronic from main excretory duct of the parotid and submandibular salivary gland diseases. This study reports the first gland or by collection of saliva in the area of the main duct experiences with scintigraphic analyses of salivary gland orifices. However, these methods are characterised by high function in a rat model. Materials and Methods: Anatomical inter-observer variability and low reproducibility. and scintigraphic studies were performed for topographic Salivary gland scintigraphy, first described by Boerner et al. differentiation of major salivary glands of Wistar rats. in 1965, is meanwhile a standard procedure in the clinical 99mtechnetium pertechnetate salivary gland scanning was routine (3, 10). -

Modality Tables for PS One 2019
PowerScribe One Modality Tables Product: PowerScribe One Doc. Num: PS1A023 Version: 2019 Date: 22 March 2019 Subject: Modality Tables Overview The table in this document lists the modalities that are available for categorization in PowerScribe® One. It is very important to spell these items as they are shown in this document when creating a spreadsheet to map your procedure codes to the Nuance modalities. Note: Some of the modalities in this table contain one or more levels of sub-categories, which appear in the columns to the right of the main item. In the PowerScribe One Administrator Portal, (under Setup > Procedures) a category that has a small plus sign (+) to the left of it contains additional sub-categories. Note: Peer Review customers must be aware that only certain modalities are supported by the American College of Radiology (ACR). If you are going to use the Send to ACR feature within the peer review application, you must map your applicable procedures to the supported ACR modalities, as indicated in the right-hand column of the table. © 2019 Nuance Communications, Inc. All rights reserved. Modality Tables Modality Table Modality Sub-Category 1 Sub-Category 2 Supported by ACR? Absorptiometry No DEXA No X-Ray - Absorptiometry Yes Conventional No Tomography CT Yes Calcium Scoring Yes CTA Yes Echocardiography Yes EEG No ETT No Fluoroscopy No Fluoroscopy - Dynamic No Invasive Yes Angiography Yes Angioplasty Yes Arterial Procedure Yes Arthrography Yes Atherectomy Yes Bronchography Yes Cardiac Procedure Yes Catheter Drainage Yes -

Infrequently Performed Studies in Nuclear Medicine: Part 2*
Infrequently Performed Studies in Nuclear Medicine: Part 2* Anita MacDonald and Steven Burrell Department of Diagnostic Radiology, Queen Elizabeth II Health Sciences Centre and Dalhousie University, Halifax, Nova Scotia, Canada clinical and technical aspects of each study, along with a Nuclear medicine is a diverse medical field, encompassing a brief comparison of alternative modalities that may also be wide range of studies. Many studies are performed on a daily ba- used, are discussed. sis, whereas some nuclear medicine studies are performed on a more infrequent basis. The following article is the second article CSF SHUNT IMAGING in a 2-part series on infrequently performed studies in nuclear medicine. The procedures reviewed include CSF shunt imaging, Hydrocephalus is an overaccumulation of CSF in the brain death scintigraphy, testicular scanning, quantitative lung ventricles of the brain. Many reasons exist for the devel- perfusion studies, lymphoscintigraphy, and salivary gland scin- opment of hydrocephalus in a patient, including an over- tigraphy. This article may be of use to technologists faced with production of CSF, an obstruction of CSF flow, and the task of performing one of these uncommon studies. After reviewing the article, the reader should be able to list the indica- problems with reabsorption of the CSF. Broadly speaking, tions for each study, list the recommended doses, describe the hydrocephalus may be classified as communicating hydro- proper method of dose administration for each study, discuss cephalus and noncommunicating hydrocephalus. patient preparation for each procedure, and describe issues Communicating hydrocephalus occurs when there is that may arise when performing these studies. patent communication between the ventricles but impair- Key Words: infrequently performed studies; CSF shunt; brain ment of CSF reabsorption. -

Hypoplasia of the Parotid Gland: Computed Tomography Sialography Diagnosis
Vol. 116 No. 4 October 2013 Hypoplasia of the parotid gland: computed tomography sialography diagnosis Lisha Sun, PhD,a Zhipeng Sun, MD,b and Xuchen Ma, PhDc School and Hospital of Stomatology, Peking University, Beijing, China We report two unusual cases of non-syndromic hypoplasia of the parotid gland. The hypoplastic parotid gland mimicked a preauricular parotid tumor in one case and presented as an incidental image finding in the other case. Absence of the deep lobe and isthmus of the parotid parenchyma could be determined on axial computed tomography (CT) by revealing fat tissue composition of the parotid space. The underdeveloped superficial lobe of the gland was observed on three- dimensional CT sialography. (Oral Surg Oral Med Oral Pathol Oral Radiol 2013;116:e297-e301) The parotid gland is the largest salivary gland and is development due to the absence of primordial tissue. anatomically divided into the superficial and deep lobes Hypoplasia is featured by underdevelopment or incom- by the facial nerve and its branches. The superficial lobe plete formation of a tissue or organ, which is similar to extends anteriorly superficial to the masseter muscle aplasia but less severe. Hypoplasia of the parotid gland and posteriorly to the retromandibular parotid space. may occur per se or associated with hypoplasia or aplasia Cranially the superficial lobe extends to the zygomatic of the submandibular gland and lacrimal gland.5 arch and caudally to the angle of the mandible. The Imaging methods of ultrasonography, X-ray sialog- Stensen’s duct emerges from the anterior superficial raphy, computed tomography (CT), magnetic resonance lobe of the parotid gland and courses over the masseter imaging (MRI) and scintigraphy could detect the muscle. -

Manual on Contrast Media Version 7 2010
MANUAL ON CONTRAST MEDIA VERSION 7 2010 Quality is our Image™ Table of Contents TOPIC Preface ............................................................................................................................... Introduction ....................................................................................................................... Patient Selection and Preparation Strategies ...................................................................... Injection of Contrast Media ................................................................................................ Extravasation of Contrast Media ....................................................................................... Incidence of Adverse Effects .............................................................................................. Adverse Effects of Iodinated Contrast Media .................................................................... Contrast Nephrotoxicity ..................................................................................................... Metformin ........................................................................................................................ Contrast Media in Children ............................................................................................... Iodinated Gastrointestinal Contrast Media: Indications and Guidelines ............................. Adverse Reactions to Gadolinium-Based Contrast Media ................................................. Nephrogenic Systemic Fibrosis -

New Zealand Data Sheet, Iomeron®
NEW ZEALAND DATA SHEET, IOMERON® 1 PRODUCT NAME IOMERON® (iomeprol) solution for injection 300 mg iodine per mL IOMERON® (iomeprol) solution for injection 350 mg iodine per mL IOMERON® (Iomeprol) solution for injection 400 mg iodine per mL 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Active substance: Iomeprol 612 mg / mL (equivalent to 300 mg iodine per mL) Iomeprol 714 mg / mL (equivalent to 350 mg iodine per mL) Iomeprol 816 mg (equivalent to 400 mg iodine per mL) Excipients: Trometamol (tromethamine USP), hydrochloric acid (Ph.Eur.), water for injection (Ph.Eur.). 3 PHARMACEUTICAL FORM Solution for injection. 4 CLINICAL PARTICULARS 4.1 Therapeutic indications IOMERON® contains as its active principal iomeprol, a tri-iodinated, non-ionic contrast agent, and is for use in X ray examinations: Iomeron 300: Intravenous urography (in adults and paediatrics), peripheral phlebography, CT (brain and body), cavernosography, intravenous DSA, conventional angiography, intra-arterial DSA, angiocardiography (in adults and paediatrics), conventional selective coronary arteriography, interventional coronary arteriography, ERCP, arthrography, hysterosalpingography, fistulography, discography, galactography, cholangiography, dacryocystography, sialography, retrograde urethrography, retrograde pyelo-ureterograpy, myelography. Iomeron 350: Intravenous urography (in adults and paediatrics), CT (body), intravenous DSA, conventional angiography, intra-arterial DSA, angiocardiography (in adults and paediatrics), conventional selective coronary arteriography, -

Administration of Medicinal Products By
Guidance for the Administration of Medicinal Products by non-medical personnel as part of clinical nuclear medicine procedures Report May 2016 British Nuclear Medicine Society Professional Standards Committee 1 Contents Introduction……………………………………………………………………………3 Local Protocols……………………………………………………………………….4 Preparation of medicines…………………………………….………………………4 Administration of medicines………………………………………………………....5 Medicines used in nuclear medicine as part of the clinical procedure (2016)…………………………………………………………….……………………6 References…………………………………………………,,,,,,,,,,,,,,,,,,,,,,,,,,,,,……7 Individual medicine templates Adenosine…………………………………………………………….……...8 Atropine……………………………………………………………………..10 Cimetidine…………………………………………………………………..12 Diazepam………………………………………………………………...…16 Dipyridamole………………………………………………………………..19 Dobutamine………………………………………………………………...22 Furosemide…………………………………………………………………26 Morphine…………………………………………………………………….32 Omeprazole…………………………………………………………………34 Ranitidine……………………………………………………………………37 Regadenoson…………………………………………………………...….40 X-ray contrast media Iohexol (Omnipaque)………………………………………………….......44 Iodixanol (Visipaque) ……………………………………………………..48 Loversol (Optiray)……………………………………………………….…54 Iomeprol (Iomeron)………………………………………………………...62 Lopamidol (Niopam)……………………………………………………….69 Lobitridol (Xenetix)…………………………………………………………77 2 Introduction A number of nuclear medicine procedures require the administration of non- radioactive medicinal products in order to optimise the biodistribution of the radiopharmaceutical. The preparation