Administration of Medicinal Products By
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

AHFS Pharmacologic-Therapeutic Classification System
AHFS Pharmacologic-Therapeutic Classification System Abacavir 48:24 - Mucolytic Agents - 382638 8:18.08.20 - HIV Nucleoside and Nucleotide Reverse Acitretin 84:92 - Skin and Mucous Membrane Agents, Abaloparatide 68:24.08 - Parathyroid Agents - 317036 Aclidinium Abatacept 12:08.08 - Antimuscarinics/Antispasmodics - 313022 92:36 - Disease-modifying Antirheumatic Drugs - Acrivastine 92:20 - Immunomodulatory Agents - 306003 4:08 - Second Generation Antihistamines - 394040 Abciximab 48:04.08 - Second Generation Antihistamines - 394040 20:12.18 - Platelet-aggregation Inhibitors - 395014 Acyclovir Abemaciclib 8:18.32 - Nucleosides and Nucleotides - 381045 10:00 - Antineoplastic Agents - 317058 84:04.06 - Antivirals - 381036 Abiraterone Adalimumab; -adaz 10:00 - Antineoplastic Agents - 311027 92:36 - Disease-modifying Antirheumatic Drugs - AbobotulinumtoxinA 56:92 - GI Drugs, Miscellaneous - 302046 92:20 - Immunomodulatory Agents - 302046 92:92 - Other Miscellaneous Therapeutic Agents - 12:20.92 - Skeletal Muscle Relaxants, Miscellaneous - Adapalene 84:92 - Skin and Mucous Membrane Agents, Acalabrutinib 10:00 - Antineoplastic Agents - 317059 Adefovir Acamprosate 8:18.32 - Nucleosides and Nucleotides - 302036 28:92 - Central Nervous System Agents, Adenosine 24:04.04.24 - Class IV Antiarrhythmics - 304010 Acarbose Adenovirus Vaccine Live Oral 68:20.02 - alpha-Glucosidase Inhibitors - 396015 80:12 - Vaccines - 315016 Acebutolol Ado-Trastuzumab 24:24 - beta-Adrenergic Blocking Agents - 387003 10:00 - Antineoplastic Agents - 313041 12:16.08.08 - Selective -

Research Article Magnetic Resonance Sialography Findings of Submandibular Ducts Imaging
Hindawi Publishing Corporation BioMed Research International Volume 2013, Article ID 417052, 6 pages http://dx.doi.org/10.1155/2013/417052 Research Article Magnetic Resonance Sialography Findings of Submandibular Ducts Imaging Nezahat Karaca ErdoLan,1 Canan Altay,2 Nesibe Özenler,3 TuLba Bozkurt,1 Engin Uluç,1 Berna Dirim Mete,1 and Esmail Özdemir4 1 Department of Radiology, Izmir Ataturk¨ Research and Training Hospital, Basın Sitesi, Karabaglar,˘ 35360 Izmir, Turkey 2 Department of Radiology, Medical School, Dokuz Eylul University, Inciralti, 35340 Izmir, Turkey 3 Department of Radiology, Balıkesir Ataturk¨ State Hospital, Yıldız Mahallesi Soma Caddesi No. 1, 10100 Balıkesir, Turkey 4 Universal Ege Health Hospital, 35220 Izmir, Turkey Correspondence should be addressed to Canan Altay; [email protected] Received 2 April 2013; Revised 29 May 2013; Accepted 12 June 2013 Academic Editor: Yoshito Tsushima Copyright © 2013 Nezahat Karaca Erdogan˘ et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Purpose. We aimed to assess the problem solving capability of magnetic resonance sialography (MR sialography), a noninvasive method for imaging submandibular gland ducts and determining duct-related pathologies, by comparing diseased and healthy cases. Materials and Methods. We conducted radiological assessment on a total of 60 submandibular glands (mean age 44.7) in 20 cases and 10 volunteers. MR sialography examinations were conducted with single-shot fast spin-echo sequence by using a surface coil placed on the submandibular gland. Each gland was evaluated in terms of the length, width and stricture of the main duct, as well as the difference between the intraparenchymal duct width, and the main duct width. -

Health Plan Insights
Health Plan Insights January 2020 Updates from December 2019 800.361.4542 | envisionrx.com Confidential - Document has confidential information and may not be copied, published or distributed, in whole or in part, in any form or medium, without EnvisionRxOptions’ prior written consent. Recent FDA Approvals New Medications TRADE NAME DOSAGE FORM APPROVAL MANUFACTURER INDICATION(S) (generic name) STRENGTH DATE Avsola Amgen Inc. Injection, Biosimilar to Remicade. For the treatment December 6, 2019 (infliximab-axxq) 100 mg/20 mL of/reducing the signs and symptoms of: Crohn’s disease, pediatric Crohn’s disease, ulcerative colitis, rheumatoid arthritis in combination with methotrexate, psoriatic arthritis, and plaque psoriasis. Vyondys 53 Sarepta Intravenous Solution, For the treatment of Duchenne muscular December 12, (golodirsen) Therapeutics, Inc. 50 mg/mL dystrophy (DMD) in patients who have a 2019 confirmed mutation of the DMD gene that is amenable to exon 53 skipping. Padcev Astellas Injection, For the treatment of adult patients with locally December 18, (enfortumab 20 mg/vial and 30 advanced or metastatic urothelial cancer who 2019 vedotin-ejfv) mg/vial have previously received a programmed death receptor-1 (PD-1) or programmed death-ligand 1 (PD-L1) inhibitor, and a platinum-containing chemotherapy in the neoadjuvant/adjuvant, locally advanced or metastatic setting. Conjupri CSPC Ouyi Tablets, For use alone or in combination with other (levamlodipine) Pharmaceutical 1.25 mg, 2.5 mg, and antihypertensive agents for the treatment of December 19, Co., Ltd. 5 mg hypertension, to lower blood pressure. 2019 Caplyta Intra-Cellular Capsules, For the treatment of schizophrenia in adults. December 20, (lumateperone) Therapies, Inc. -
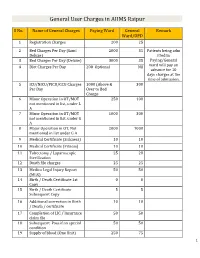
General User Charges in AIIMS Raipur
General User Charges in AIIMS Raipur S No. Name of General Charges Paying Ward General Remark Ward/OPD 1 Registration Charges 200 25 2 Bed Charges Per Day (Sami 2000 35 Patients being adm Deluxe) itted in 3 Bed Charges Per Day (Deluxe) 3000 35 Paying/General 4 Diet Charges Per Day 200 Optional Nil ward will pay an advance for 10 days charges at the time of admission. 5 ICU/NICU/PICU/CCU Charges 1000 (Above & 300 Per Day Over to Bed Charge 6 Minor Operation in OT/MOT 250 100 not mentioned in list, under L A 7 Minor Operation in OT/MOT 1000 300 not mentioned in list, under G A 8 Major Operation in OT, Not 2000 1000 mentioned in list under G A 9 Medical Certificate (Sickness) 10 10 10 Medical Certificate (Fitness) 10 10 11 Tubectomy / Laparoscopic 25 20 Sterilization 12 Death file charges 25 25 13 Medico Legal Injury Report 50 50 (MLR) 14 Birth / Death Certificate 1st 0 0 Copy 15 Birth / Death Certificate 5 5 Subsequent Copy 16 Additional correction in Birth 10 10 / Death / certificate 17 Completion of LIC / Insurance 50 50 claim file 18 Subsequent Pass if on special 50 50 condition 19 Supply of blood (One Unit) 250 75 1 20 Medical Board Certificate 500 500 On Special Case User Charges for Investigations in AIIMS Raipur S No. Name of Investigations Paying General Remark Ward Ward/OPD Anaesthsia 1 ABG 75 50 2 ABG ALONGWITH 150 100 ELECTROLYTES(NA+,K+)(Na,K) 3 ONLY ELECTROLYTES(Na+,K+,Cl,Ca+) 75 50 4 ONLY CALCIUM 50 25 5 GLUCOSE 25 20 6 LACTATE 25 20 7 UREA. -

ACR Appropriateness Criteria® Right Upper Quadrant Pain
Revised 2018 American College of Radiology ACR Appropriateness Criteria® Right Upper Quadrant Pain Variant 1: Right upper quadrant pain. Suspected biliary disease. Initial imaging. Procedure Appropriateness Category Relative Radiation Level US abdomen Usually Appropriate O CT abdomen with IV contrast May Be Appropriate ☢☢☢ MRI abdomen without and with IV May Be Appropriate contrast with MRCP O MRI abdomen without IV contrast with May Be Appropriate MRCP O Nuclear medicine scan gallbladder May Be Appropriate ☢☢ CT abdomen without IV contrast May Be Appropriate ☢☢☢ CT abdomen without and with IV Usually Not Appropriate contrast ☢☢☢☢ Variant 2: Right upper quadrant pain. No fever or high white blood cell (WBC) count. Suspected biliary disease. Negative or equivocal ultrasound. Procedure Appropriateness Category Relative Radiation Level MRI abdomen without and with IV Usually Appropriate contrast with MRCP O CT abdomen with IV contrast Usually Appropriate ☢☢☢ MRI abdomen without IV contrast with Usually Appropriate MRCP O Nuclear medicine scan gallbladder May Be Appropriate ☢☢ CT abdomen without IV contrast May Be Appropriate ☢☢☢ CT abdomen without and with IV Usually Not Appropriate contrast ☢☢☢☢ Variant 3: Right upper quadrant pain. Fever, elevated WBC count. Suspected biliary disease. Negative or equivocal ultrasound. Procedure Appropriateness Category Relative Radiation Level MRI abdomen without and with IV Usually Appropriate contrast with MRCP O CT abdomen with IV contrast Usually Appropriate ☢☢☢ Nuclear medicine scan gallbladder Usually Appropriate ☢☢ MRI abdomen without IV contrast with May Be Appropriate MRCP O CT abdomen without IV contrast May Be Appropriate ☢☢☢ CT abdomen without and with IV Usually Not Appropriate contrast ☢☢☢☢ ACR Appropriateness Criteria® 1 Right Upper Quadrant Pain Variant 4: Right upper quadrant pain. -

Cholecystokinin Cholescintigraphy: Methodology and Normal Values Using a Lactose-Free Fatty-Meal Food Supplement
Cholecystokinin Cholescintigraphy: Methodology and Normal Values Using a Lactose-Free Fatty-Meal Food Supplement Harvey A. Ziessman, MD; Douglas A. Jones, MD; Larry R. Muenz, PhD; and Anup K. Agarval, MS Department of Radiology, Georgetown University Hospital, Washington, DC Fatty meals have been used by investigators and clini- The purpose of this investigation was to evaluate the use of a cians over the years to evaluate gallbladder contraction in commercially available lactose-free fatty-meal food supple- conjunction with oral cholecystography, ultrasonography, ment, as an alternative to sincalide cholescintigraphy, to de- and cholescintigraphy. Proponents assert that fatty meals velop a standard methodology, and to determine normal gall- are physiologic and low in cost. Numerous different fatty bladder ejection fractions (GBEFs) for this supplement. meals have been used. Many are institution specific. Meth- Methods: Twenty healthy volunteers all had negative medical histories for hepatobiliary and gallbladder disease, had no per- odologies have differed, and few investigations have stud- sonal or family history of hepatobiliary disease, and were not ied a sufficient number of subjects to establish valid normal taking any medication known to affect gallbladder emptying. All GBEFs for the specific meal. Whole milk and half-and-half were prescreened with a complete blood cell count, compre- have the advantage of being simple to prepare and admin- hensive metabolic profile, gallbladder and liver ultrasonography, ister (4–7). Milk has been particularly well investigated. and conventional cholescintigraphy. Three of the 20 subjects Large numbers of healthy subjects have been studied, a were eliminated from the final analysis because of an abnormal- clear methodology described, and normal values determined ity in one of the above studies. -

Procedure Codes for Physician: Radiology
NEW YORK STATE MEDICAID PROGRAM PHYSICIAN - PROCEDURE CODES SECTION 4 - RADIOLOGY Physician – Procedure Codes, Section 4 - Radiology Table of Contents GENERAL INSTRUCTIONS ............................................................................................................ 4 GENERAL RULES AND INFORMATION ......................................................................................... 6 MMIS RADIOLOGY MODIFIERS .................................................................................................... 8 DIAGNOSTIC RADIOLOGY (DIAGNOSTIC IMAGING)................................................................. 9 HEAD AND NECK.................................................................................................................... 9 CHEST .................................................................................................................................. 10 SPINE AND PELVIS .............................................................................................................. 11 UPPER EXTREMITIES .......................................................................................................... 12 LOWER EXTREMITIES ......................................................................................................... 13 ABDOMEN ............................................................................................................................ 14 GASTROINTESTINAL TRACT ............................................................................................... 15 URINARY -

The Diagnosis of Acute Cholecystitis: Sensitivity of Sonography
The Diagnosis of Acute Cholecystitis: Sensitivity of Sonography, Cholescintigraphy and Computed Tomography Patthisak Changphaisarnkul MD*, Supakajee Saengruang-Orn PhD*, Trirat Boonya-Asadorn MD* * Division of Radiology, Phramonkutklao Hospital, Bangkok, Thailand Objective: To compare the sensitivity of sonographic, cholescintigraphic, and computed tomographic examination of acute cholecystitis to the pathology result, which is considered the Gold Standard. Material and Method: A retrospective analytic study was conducted among 412 patients, aged between 15 and 98 years, who underwent cholecystectomy surgeries, and whose pathology results indicated acute cholecystitis between July 2004 and May 2013. The sensitivity and the differences between sensitivity of the three methods were calculated in all patients. Complicated acute cholecystitis cases were analyzed separately. Results: The three methods demonstrated statistically significant differences in sensitivity (p-value = 0.017), with the cholescintigraphy as the most sensitive method (84.2%), followed by computed tomography (67.3%), and sonography (59.8%). Concerning the samples with the pathology result indicating complicated acute cholecystitis, computed tomography was statistically significantly more sensitive than sonography in detecting acute cholecystitis, whether or not the complications were identified (100% and 63.6%, respectively, with p-value = 0.0055). None of the patients with the pathology result of complicated acute cholecystitis case was examined by cholescintigraphy, thus, no calculation was possible. Regarding the ability to detect the complications of acute cholecystitis, computed tomography had a sensitivity of 35.71% (5 in 14 patients), while sonographic examinations could not detect any of the complications. Conclusion: Cholescintigraphy is a more sensitive method than computed tomography and sonography, but the three methods have its own advantages, disadvantages, and limitations, which must be considered for each individual patient. -

Insight ISSUE 1
ISSUE 1 IMMUNE CHECKPOINTS & insight IMMUNOTHERAPY RESEARCH Immune Checkpoints and Cancer Cancer immunotherapy seeks to use the many A better approach is to intervene when T cells components of the immune system to attack meet cancer cells, where TCR-mediated activation cancer cells. More specifically, immunotherapy initiates cell killing. Programmed death-1 (PD-1) is maximizes the effectiveness of components of a lymphocyte receptor that binds PD-L1 or PD-L2. the antigen-presentation and antigen-response When PD-L1 is expressed on cancer cells, it causes system, primarily dendritic cells and lymphocytes, PD-1 to negatively regulate TCR-mediated activation respectively. Ideally, this approach can offer more of T cells, limiting their cytotoxic activity. Several selective killing of cancer cells than other therapeutic antibodies have been developed to block the modalities, such as chemotherapy. ability of PD-L1 to interact with PD-1. Clinical trials using these antibodies to antagonize the PD-L1/PD-1 Immune checkpoint therapy is a form of cancer interaction have demonstrated tumor killing that is immunotherapy that centers on lymphocyte both specific and long-lasting.3,4 In May 2016, signaling, with a current focus on T cells. These the first PD-L1 inhibitor was approved by the cells can be activated to multiply, secrete cytokines, U.S. Food and Drug Administration for the and kill target cells with high selectivity. Activation treatment of bladder cancer. requires the T cell receptor (TCR) be stimulated by an antigen presented by the major histocompatibility Studies using antibodies to block the inhibitory complex (Ag/MHC). Selectivity and strength of checkpoint receptors CTLA-4 and PD-1 demonstrate activation are regulated by co-stimulatory or the feasibility of this type of immunotherapy. -

© Ferrata Storti Foundation
LETTERS TO THE EDITOR It was recently demonstrated in sickle cell mice that Impaired pulmonary endothelial barrier function in increased vascular permeability contributes to pulmonary sickle cell mice edema and the pathophysiology of ACS.8 Studies using Evans blue dye confirmed an increased permeability in Acute and chronic pulmonary complications leading to the sickle cell mouse lung, however, EC barrier function significant morbidity and mortality occur in persons with was not investigated. To gain additional insights into bar- sickle cell disease (SCD). One of the leading causes of rier function, we performed studies with cultured EC 1 death is acute chest syndrome (ACS), diagnosed by a from the lungs of the Townes knock-in sickle cell mouse 2 new infiltrate on chest x-ray often triggered by infection. (SS) and heterozygote (AS) littermates. Using endothe- The resulting low oxygen saturation leads to hemoglobin lial-specific CD31 conjugated Dynabeads, we isolated S polymerization, red blood cell sickling, vaso-occlusion mouse lung microvascular EC (MLMVEC) from SS 3 and hypoxia, the hallmark of ACS. Pulmonary endothe- (SS-MLMVEC) and AS (AS-MLMVEC) mice (Online lial cell (EC) barrier function is mainly regulated by the Supplementary Methods). The EC were grown as a opening and closing of tight junctions in the intercellular monolayer, and phase-contrast microscopy demonstrated space that controls the passage of macromolecules and the cobblestone structure (Figure 1A). They expressed cells across the vascular wall.4 The association of tight endothelial nitric oxide synthase and platelet adhesion junctions with the actin cytoskeleton is required for the molecule and take up acetylated low density lipoprotein dynamic regulation of junction opening and closure.5 (data not shown), characteristics consistent with the EC Under hypoxic and infection conditions, cell-cell junc- phenotype. -

What Should I Know About My Cardiac Nuclear Stress Test with Lexiscan® (Regadenoson) Injection?
What should I know about my cardiac nuclear stress test with Lexiscan® (regadenoson) injection? Use: Lexiscan (regadenoson) injection is a prescription drug given through an IV line that increases blood flow through the arteries of the heart during a cardiac nuclear stress test. Lexiscan is given to patients when they are unable to exercise adequately for a stress test. Important Safety Information: Lexiscan should not be given to patients who have certain abnormal heart rhythms unless they have a pacemaker. PLEASE SEE IMPORTANT SAFETY INFORMATION ON PAGE 16. PLEASE SEE FULL PRESCRIBING INFORMATION ON PAGES 22-25. Coronary artery disease What is coronary artery disease? The heart is a specialized muscle. Like other muscles in your body, it needs oxygen and nutrients. The coronary (heart) arteries deliver oxygen and nutrients to your heart so that it can effectively pump blood throughout your body. People with heart disease may have 1 or more coronary arteries that have become narrowed or clogged over time by fatty deposits (also called plaques). These can decrease blood flow to the heart. Page 2 is an illustration that shows you what a healthy artery looks like compared with an unhealthy artery. So less blood flow is bad, right? Exactly. Reduced blood flow may cause chest pain (angina), shortness of normal artery breath, and potentially a heart attack. Injured heart muscle can be permanently damaged if the coronary arteries stay blocked for too long. If there is a problem with your heart, it is important to find out about it as soon as possible. My doctor scheduled me for an MPI test. -

Rxoutlook® 1St Quarter 2019
® RxOutlook 1st Quarter 2020 optum.com/optumrx a RxOutlook 1st Quarter 2020 Orphan drugs continue to feature prominently in the drug development pipeline In 1983 the Orphan Drug Act was signed into law. Thirty seven years later, what was initially envisioned as a minor category of drugs has become a major part of the drug development pipeline. The Orphan Drug Act was passed by the United States Congress in 1983 in order to spur drug development for rare conditions with high unmet need. The legislation provided financial incentives to manufacturers if they could demonstrate that the target population for their drug consisted of fewer than 200,000 persons in the United States, or that there was no reasonable expectation that commercial sales would be sufficient to recoup the developmental costs associated with the drug. These “Orphan Drug” approvals have become increasingly common over the last two decades. In 2000, two of the 27 (7%) new drugs approved by the FDA had Orphan Designation, whereas in 2019, 20 of the 48 new drugs (42%) approved by the FDA had Orphan Designation. Since the passage of the Orphan Drug Act, 37 years ago, additional regulations and FDA designations have been implemented in an attempt to further expedite drug development for certain serious and life threatening conditions. Drugs with a Fast Track designation can use Phase 2 clinical trials to support FDA approval. Drugs with Breakthrough Therapy designation can use alternative clinical trial designs instead of the traditional randomized, double-blind, placebo-controlled trial. Additionally, drugs may be approved via the Accelerated Approval pathway using surrogate endpoints in clinical trials rather than clinical outcomes.