Other Primate Species* A
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Development Team
Paper No. : 14 Human Origin and Evolution Module : 09 Classification and Distribution of Living Primates Development Team Principal Investigator Prof. Anup Kumar Kapoor Department of Anthropology, University of Delhi Dr. Satwanti Kapoor (Retd Professor) Paper Coordinator Department of Anthropology, University of Delhi Mr. Vijit Deepani & Prof. A.K. Kapoor Content Writer Department of Anthropology, University of Delhi Prof. R.K. Pathak Content Reviewer Department of Anthropology, Panjab University, Chandigarh 1 Classification and Distribution of Living Primates Anthropology Description of Module Subject Name Anthropology Paper Name Human Origin and Evolution Module Name/Title Classification and Distribution of Living Primates Module Id 09 Contents: Primates: A brief Outline Classification of Living Primates Distribution of Living Primates Summary Learning Objectives: To understand the classification of living primates. To discern the distribution of living primates. 2 Classification and Distribution of Living Primates Anthropology Primates: A brief Outline Primates reside at the initial stage in the series of evolution of man and therefore constitute the first footstep of man’s origin. Primates are primarily mammals possessing several basic mammalian features such as presence of mammary glands, dense body hair; heterodonty, increased brain size, endothermy, a relatively long gestation period followed by live birth, considerable capacity for learning and behavioural flexibility. St. George J Mivart (1873) defined Primates (as an order) -

Radar.Brookes.Ac.Uk/Radar/Items/C664d3c7-1663-4D34-Aed5-E3e070598081/1
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Oxford Brookes University: RADAR RADAR Research Archive and Digital Asset Repository Pozzi, L, Nekaris, K, Perkin, A, Bearder, S, Pimley, E, Schulze, H, Streicher, U, Nadler, T, Kitchener, A, Zischler, H, Zinner, D and Roos, C Remarkable Ancient Divergences Amongst Neglected Lorisiform Primates Pozzi, L, Nekaris, K, Perkin, A, Bearder, S, Pimley, E, Schulze, H, Streicher, U, Nadler, T, Kitchener, A, Zischler, H, Zinner, D and Roos, C (2015) Remarkable Ancient Divergences Amongst Neglected Lorisiform Primates. Zoological Journal of the Linnean Society, 175 (3). pp. 661-674. doi: 10.1111/zoj.12286 This version is available: https://radar.brookes.ac.uk/radar/items/c664d3c7-1663-4d34-aed5-e3e070598081/1/ Available on RADAR: August 2016 Copyright © and Moral Rights are retained by the author(s) and/ or other copyright owners. A copy can be downloaded for personal non-commercial research or study, without prior permission or charge. This item cannot be reproduced or quoted extensively from without first obtaining permission in writing from the copyright holder(s). The content must not be changed in any way or sold commercially in any format or medium without the formal permission of the copyright holders. This document is the published version of the journal article. WWW.BROOKES.AC.UK/GO/RADAR bs_bs_banner Zoological Journal of the Linnean Society, 2015, 175, 661–674. With 3 figures Remarkable ancient divergences amongst neglected lorisiform primates LUCA -

Exam 1 Set 3 Taxonomy and Primates
Goodall Films • Four classic films from the 1960s of Goodalls early work with Gombe (Tanzania —East Africa) chimpanzees • Introduction to Chimpanzee Behavior • Infant Development • Feeding and Food Sharing • Tool Using Primates! Specifically the EXTANT primates, i.e., the species that are still alive today: these include some prosimians, some monkeys, & some apes (-next: fossil hominins, who are extinct) Diversity ...200$300&species& Taxonomy What are primates? Overview: What are primates? • Taxonomy of living • Prosimians (Strepsirhines) – Lorises things – Lemurs • Distinguishing – Tarsiers (?) • Anthropoids (Haplorhines) primate – Platyrrhines characteristics • Cebids • Atelines • Primate taxonomy: • Callitrichids distinguishing characteristics – Catarrhines within the Order Primate… • Cercopithecoids – Cercopithecines – Colobines • Hominoids – Hylobatids – Pongids – Hominins Taxonomy: Hierarchical and Linnean (between Kingdoms and Species, but really not a totally accurate representation) • Subspecies • Species • Genus • Family • Infraorder • Order • Class • Phylum • Kingdom Tree of life -based on traits we think we observe -Beware anthropocentrism, the concept that humans may regard themselves as the central and most significant entities in the universe, or that they assess reality through an exclusively human perspective. Taxonomy: Kingdoms (6 here) Kingdom Animalia • Ingestive heterotrophs • Lack cell wall • Motile at at least some part of their lives • Embryos have a blastula stage (a hollow ball of cells) • Usually an internal -

Rhesus Macaques (Macaca Mulatta) Recognize Group Membership Via Olfactory Cues Alone
Behav Ecol Sociobiol (2015) 69:2019–2034 DOI 10.1007/s00265-015-2013-y ORIGINAL ARTICLE Rhesus macaques (Macaca mulatta) recognize group membership via olfactory cues alone Stefanie Henkel 1,2 & Angelina Ruiz Lambides1,3,4 & Anne Berger5 & Ruth Thomsen1,6,7 & Anja Widdig1,3 Received: 9 April 2015 /Revised: 18 September 2015 /Accepted: 21 September 2015 /Published online: 31 October 2015 # Springer-Verlag Berlin Heidelberg 2015 Abstract The ability to distinguish group members from con- paradigm to investigate whether rhesus macaques (Macaca specifics living in other groups is crucial for gregarious spe- mulatta) can discriminate between body odors of female cies. Olfaction is known to play a major role in group recog- group members and females from different social groups. nition and territorial defense in a wide range of mammalian We conducted the study on the research island Cayo Santiago, taxa. Although primates have been typically regarded as Puerto Rico, in the non-mating season and controlled for kin- microsmatic (having a poor sense of smell), increasing evi- ship and familiarity using extensive pedigree and demograph- dence suggests that olfaction may play a greater role in pri- ic data. Our results indicate that both males and females in- mates’ social life than previously assumed. In this study, we spect out-group odors significantly longer than in-group carried out behavioral bioassays using a signaler-receiver odors. Males licked odors more often than females, and older animals licked more often than younger ones. Furthermore, individuals tended to place their nose longer towards odors Communicated by E. Huchard when the odor donor’s group rank was higher than the rank of Electronic supplementary material The online version of this article their own group. -

Dorsorostral Snout Muscles in the Rat Subserve Coordinated Movement for Whisking and Sniffing
THE ANATOMICAL RECORD 000:000–000 (2012) Dorsorostral Snout Muscles in the Rat Subserve Coordinated Movement for Whisking and Sniffing SEBASTIAN HAIDARLIU,1* DAVID GOLOMB,2,3 DAVID KLEINFELD,4 1 AND EHUD AHISSAR 1Department of Neurobiology, The Weizmann Institute of Science, Rehovot, Israel 2Department of Physiology and Zlotowski Center for Neuroscience, Ben-Gurion University of the Negev, Beer-Sheva, Israel 3Janelia Farm Research Campus, Howard Hughes Medical Institute, Ashburn, Virginia 4Department of Physics and Section of Neurobiology, University of California, San Diego, La Jolla, California ABSTRACT Histochemical examination of the dorsorostral quadrant of the rat snout revealed superficial and deep muscles that are involved in whisk- ing, sniffing, and airflow control. The part of M. nasolabialis profundus that acts as an intrinsic (follicular) muscle to facilitate protraction and translation of the vibrissae is described. An intraturbinate and selected rostral-most nasal muscles that can influence major routs of inspiratory airflow and rhinarial touch through their control of nostril configuration, atrioturbinate and rhinarium position, were revealed. Anat Rec, 00:000– 000, 2012. VC 2012 Wiley Periodicals, Inc. Key words: active sensing; breathing; nose muscles; rodents Rodents with poor vision and nocturnal activity rely feld, 2003; Hill et al., 2008), the specific actions of many heavily on senses of touch and smell. In the rat, whisk- of these muscles remain unclear. ing and sniffing are iterative cyclic motor features that According to the map of muscles in the MP described accompany active tactile and olfactory sensing, respec- by Dorfl€ (1982), and to known mechanical models of the tively. The whisking and sniffing rhythms can couple rat MP (Wineski, 1985; Hill et al., 2008; Simony et al., during exploratory behavior (Welker, 1964; Komisaruk, 2010; Haidarliu et al., 2011), each large vibrissa is pro- 1970; Kepecs et al., 2007; Wachowiak, 2011; Descheˆnes tracted by two intrinsic muscles (IMs). -

Aspects of Ecology and Conservation of the Pygmy Loris Nycticebus Pygmaeus in Vietnam
Aus dem Institut für Zoologie, Fischkrankheiten und Fischereibiologie der Tierärztlichen Fakultät der Ludwig-Maximilians-Universität München Angefertigt am Endangered Primate Rescue Center Cuc Phuong National Park Vietnam Aspects of Ecology and Conservation of the Pygmy Loris Nycticebus pygmaeus in Vietnam Inaugural-Dissertation zur Erlangung der tiermedizinischen Doktorwürde der Tierärztlichen Fakultät der Ludwig-Maximilians Universität München vorgelegt von Ulrike Streicher aus Bamberg München, Oktober 2004 Dem Andenken meines Vaters Preface The first pygmy lorises came to the Endangered Primate Rescue Center in 1995 and were not much more than the hobby of the first animal keeper, Manuela Klöden. They were at that time, even by Vietnamese scientists or foreign primate experts, considered not very important. They were abundant in the trade and there was little concern about their wild status. It has often been the fate of animals that are considered common not to be considered worth detailed studies. But working with confiscated pygmy lorises we discovered a number of interesting facts about them. They seasonally changed the pelage colour, they showed regular weight variations, and they did not eat in certain times of the year. And I met people interested in lorises and told them, what I had observed and realized these facts were not known. So I started to collect data more or less to proof what we had observed at the centre. Due to the daily veterinary tasks data collection was rather randomly and unfocussed. But the more we got to know about the pygmy lorises, the more interesting it became. The answer to one question immediately generated a number of consecutive questions. -

Infant Development in the Slender Loris (Loris Lydekkerianus Lydekkerianus)
RESEARCH ARTICLES Infant development in the slender loris (Loris lydekkerianus lydekkerianus) Sindhu Radhakrishna1,* and Mewa Singh Department of Psychology, University of Mysore, Mysore 570 006, India 1Present address: National Institute of Advanced Studies, Indian Institute of Science Campus, Bangalore 560 012, India lorises and bushbabies)1, the lorids (angwantibos, pottos In this article we present data on infant development in wild slender loris, a nocturnal primate species. The and lorises) typically have lower developmental and repro- ductive rates in comparison to similar-sized galagos (bush- behavioural ecology of the grey slender loris Loris lyde- 2–4 kkerianus lydekkerianus, a nocturnal strepsirrhine, babies) . Litter size in lorids and galagos appears to be was studied for 21 months (October 1997–June 1999) linked to the stability of the habitat more than body size: in a scrub jungle in Dindigul, south India. A total of species occupying harsher environments tend to have 22,834 scans were collected during 2656 h of observa- higher reproductive rates3,5. Strepsirrhine neonates are tion on identified and unidentified lorises using in- carried for short periods following birth and mode of stantaneous point and ad libitum sampling methods. infant transport can be oral (Galagos bushbabies, Cheiro- Developmental schedules were observed for twelve in- galeus dwarf lemurs, Microcebus mouse lemurs) or the dividuals born during the course of the study period. abdominal fur of the mother (Loris slender loris)3,6,7. In- A greater number of twin births were observed than fant parking is typical of the Loriformes, wherein infants singleton births and more isosexuals than heterosexu- als. -
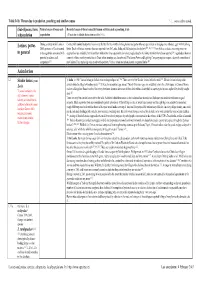
Table 14 B: Threat Due to Predation, Poaching and Similar Causes 1, 2,
Table 14 b: Threat due to predation, poaching and similar causes 1, 2, ... : source, author quoted. (Sub-)Species, form, Natural causes of losses such Recorded causes of threat caused by human activities such as poaching, trade subpopulation as predation (Threat due to habitat destruction see table 14 c) Lorises, pottos During a survey in Sri Lanka in Contact with uninsulated power lines is may be fatal for the slowly climbing lorises and pottos who are specialized on bridging over substrate gaps with their long 2001, presence of Loris seemed limbs. Death of lorises on power lines are reported from Sri Lanka, India and Malaysia (see also below) 207, 66, 211. From Africa, no data concerning pottos or in general to be negatively associated with angwantibos are available, but the problem with power lines apparently also exists, regular deaths of colobus monkeys have been reported 208, regarded as the main potential predators and cause of colobus monkey mortalities in Diani; other monkeys are also affected. The Kenya Power and Lighting Company engineers spent a day with coworkers of competitors 211. the Colobus Trust exploring ways to solve the problem. Victims of electrocution showed amputated limbs 209. Asian lorises L I Slender lorises, genus In India, in 1981 "lorises belong to India's most endangered species" 116. There are very few Slender lorises left in the wild 121. Slender lorises belong to the Loris animals found in illegal ownership (pets) 110. It was also a popular cage animal. Twenty-five years ago, one could buy a loris for a few rupees in Chennai Moore market or Bangalore Russel market. -

Olfactory Communication in Mammals 4034
Copyright 1972. All rights reserved OLFACTORY COMMUNICATION IN MAMMALS 4034 JOHN F. EISENBERG AND DEVRA G. KLEIMAN National Zoological Park Smithsonian Institution Washington, DC INTRODUCTION In the broad perspective chemical communication has been the subject of several recent reviews (Wilson 138; Johnston, Moulton & Turk 59; Wilson 137). The present review will be more restrictive; we will deal with one as- pect of chemical communication in one class of vertebrates, i.e., olfaction in the Mammalia. For the purposes of this review, olfactory communication is defined as the process whereby a chemical signal is generated by a presumptive sender and transmitted (generally through the air) to a presumptive receiver who by means of adequate receptors can identify, integrate, and respond (either be- haviorally or physiologically) to the signal. It is assumed that the sender-re- ceiver relationship is in some way the result of natural selection so that signal production by the sender leads to an increased likelihood that the sender or the species will benefit from the transmission of the message; the whole pro- cess of communication is subject to the pressures of natural selection. A chemical signal which serves to trigger a response in a conspecific re- ceiver is generally referred to as a pheromone (Wilson 137)•in contrast to an allomone, which is a signal used to communicate with a member of an- other species (Brown 16). By thus defining olfactory communication, we have eliminated from consideration in this review problems of food selection, habitat selection, etc, which do involve the chemical senses but which result from different sorts of interactions of the organism with the environment. -

Primate Conservation
Primate Conservation Global evidence for the effects of interventions Jessica Junker, Hjalmar S. Kühl, Lisa Orth, Rebecca K. Smith, Silviu O. Petrovan and William J. Sutherland Synopses of Conservation Evidence ii © 2017 William J. Sutherland This work is licensed under a Creative Commons Attribution 4.0 International license (CC BY 4.0). This license allows you to share, copy, distribute and transmit the work; to adapt the work and to make commercial use of the work providing attribution is made to the authors (but not in any way that suggests that they endorse you or your use of the work). Attribution should include the following information: Junker, J., Kühl, H.S., Orth, L., Smith, R.K., Petrovan, S.O. and Sutherland, W.J. (2017) Primate conservation: Global evidence for the effects of interventions. University of Cambridge, UK Further details about CC BY licenses are available at https://creativecommons.org/licenses/by/4.0/ Cover image: Martha Robbins/MPI-EVAN Bwindi Impenetrable National Park, Uganda Digital material and resources associated with this synopsis are available at https://www.conservationevidence.com/ iii Contents About this book ............................................................................................................................. xiii 1. Threat: Residential and commercial development ............................ 1 Key messages ........................................................................................................................................ 1 1.1. Remove and relocate ‘problem’ -

Wild Tigers in Captivity: a Study of the Effects of the Captive Environment on Tiger Behavior
Wild Tigers in Captivity: A Study of the Effects of the Captive Environment on Tiger Behavior Leigh Elizabeth Pitsko Thesis submitted to the Faculty of Virginia Polytechnic Institute and State University In partial fulfillment of the requirements for the degree of MASTER OF SCIENCE IN GEOGRAPHY Lisa M. Kennedy, Chair Lawrence S. Grossman Marcella J. Kelly Virginia Polytechnic Institute and State University Blacksburg, VA 25 April 2003 Keywords: Panthera tigris, environmental enrichment, stereotypies, conservation, exhibit design Copyright 2003, Leigh Elizabeth Pitsko ABSTRACT Wild Tigers in Captivity: A Study of the Effects of the Captive Environment on Tiger Behavior Leigh Elizabeth Pitsko Humans maintain wild animals in zoological parks for the purposes of education, conservation, research, and recreation. However, abnormal behaviors may develop in animals housed in human-made environments, if those environments do not allow them to carry out their natural behaviors (such as swimming, climbing, stalking, and predation). Captive environments in zoological parks often do not provide for natural behaviors due to spatial constraints and negative public reaction. Tigers (Panthera tigris) present a difficult case; they have large home ranges in the wild and natural predatory hunting behaviors that are difficult to provide for in captivity. As the numbers of wild tigers decline, captive breeding programs have become a major focus of the zoo community, which magnifies the importance of research on tiger husbandry. A body of research exists on small felids, but little, if any, has focused on tigers. This thesis presents an analysis of the effects of the captive environment on the behaviors of 18 captive Bengal and Siberian tigers in four zoological parks in Virginia and Pennsylvania. -

Evolution of the Nasal Structure in the Lower Tetrapods
AM. ZOOLOCIST, 7:397-413 (1967). Evolution of the Nasal Structure in the Lower Tetrapods THOMAS S. PARSONS Department of Zoology, University of Toronto, Toronto, Ontario, Canada SYNOPSIS. The gross structure of the nasal cavities and the distribution of the various types of epithelium lining them are described briefly; each living order of amphibians and reptiles possesses a characteristic and distinctive pattern. In most groups there are two sensory areas, one lined by olfactory epithelium with nerve libers leading to the main olfactory bulb and the other by vomeronasal epithelium Downloaded from https://academic.oup.com/icb/article/7/3/397/244929 by guest on 04 October 2021 with fibers to the accessory bulb. All amniotes except turtles have the vomeronasal epithelium in a ventromedial outpocketing of the nose, the Jacobson's organ, and have one or more conchae projecting into the nasal cavity from the lateral wall. Although urodeles and turtles possess the simplest nasal structure, it is not possible to show that they are primitive or to define a basic pattern for either amphibians or reptiles; all the living orders are specialized and the nasal anatomy of extinct orders is unknown. Thus it is impossible, at present, to give a convincing picture of the course of nasal evolution in the lower tetrapods. Despite the rather optimistic title of this (1948, squamates), Stebbins (1948, squa- paper, I shall, unfortunately, be able to do mates), Bellairs and Boyd (1950, squa- iittle more than make a few guesses about mates), and Parsons (1959a, reptiles). Most the evolution of the nose. I can and will of the following descriptions are based on mention briefly the major features of the these works, although others, specifically nasal anatomy of the living orders of cited in various places, were also used.