New Pest Response Guidelines
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Universidade Federal De Juiz De Fora Pós-Graduação Em Ciências Biológicas Mestrado Em Comportamento E Biologia Animal
UNIVERSIDADE FEDERAL DE JUIZ DE FORA PÓS-GRADUAÇÃO EM CIÊNCIAS BIOLÓGICAS MESTRADO EM COMPORTAMENTO E BIOLOGIA ANIMAL Camilla Aparecida de Oliveira Estratégia de história de vida e recaracterização morfológica Sarasinula linguaeformis (Semper, 1885) (Eupulmonata, Veronicellidae) Juiz de Fora 2019 Camilla Aparecida de Oliveira Estratégia de história de vida e recaracterização morfológica Sarasinula linguaeformis (Semper, 1885) (Eupulmonata, Veronicellidae) Dissertação apresentada ao Programa de Pós-Graduação em Ciências Biológicas, área de concentração: Comportamento e Biologia Animal da Universidade Federal de Juiz de Fora, como requisito parcial para obtenção do título de Mestre. Orientadora: Prof.ª. Drª. Sthefane D’ávila Juiz de Fora 2019 A todos que estiveram ao meu lado me apoiando e incentivando diante das dificuldades da carreira acadêmica, e incentivaram minha formação pessoal, profissional e dando-me suporte emocional. A vocês o meu eterno agradecimento! AGRADECIMENTOS Agradeço primeiramente a Deus por abençoar o meu caminho durante esse trabalho. A fé que tenho em Ti alimentou meu foco, minha força e minha disciplina. Depois aos meus amigos da Ciências Biológicas: Alexssandra Silva, Flávio Macanha, Isabel Macedo, Sue-helen Mondaini, Tayrine Carvalho, Kássia Malta e Yuri Carvalho meu eterno agradecimento, pois fizeram uma contribuição valiosa para a minha jornada acadêmica com seus conselhos, auxílio, palavras de apoio e risadas. Também agradeço a todos aqueles amigos que de forma direta ou indireta estiveram ajudando e torcendo por mim, em especial a Ana Claudia Mazetto, Ana Clara Files, Tamires Lima, Lígia Araújo, Raquel Seixas, Natália Corrêa e Carlota Augusta. Vocês foram fundamentais para minha formação. Agradeço à minha orientadora Sthefane D' ávila, que acompanhou meu percurso ao longo dos últimos anos e ofereceu uma orientação repleta de conhecimento, sabedoria e paciência. -

137-144. New Hemiplecta
Biodiversity Journal , 2012, 3 (2): 137-144 A new species of Hemiplecta Albers, 1850 (Gastropoda, Pul - monata, Ariophantidae) from Sumatra, Indonesia David P. Cilia 1* & John Abbas 2 1 29, Triq il-Palazz l-Aħmar, Santa Venera, Malta 28, Jalan Demaga Baru, Muara Angke, Jakarta Utara Pos 14450, Jakarta, Indonesia *Corresponding author, e-mail: [email protected] ABSTRACT The ariophantid Hemiplecta belerang sp. nov. from South Sumatra is described in this paper. It is compared with its closest congeners, from which it is geographically and reproductively isolated. KEY WORDS Ariophantidae; Hemiplecta belerang n. sp.; Sumatra; Indonesia. Received 03.06.2012; accepted 21.06.2012; printed 30.06.2012 INTRODUCTION d'Histoire Naturelle, Paris, France (MNHN); Na - tural History Museum, London, United Kingdom The family Ariophantidae Godwin-Austen, (NHMUK); National Museum of Natural History, 1888 is nested within the limacoid clade of pulmo - Mdina, Malta (NMNH); Zoological Department nates and is native to south-east Asia and India of Tel Aviv University, Israel (TAU); Institut für (Hausdorf, 2000). Evolutionsbiologie und Umweltwissenschaften/ The family includes the genus Hemiplecta Al - Zoologisches Museum Universität Zürich-Irchel, bers, 1850, a group of medium to large-sized Switzerland (ZMZ). ground-inhabiting snails (Boonngam et al., 2008; Morphology and anatomy. DG = dart gland; Schilthuizen, 2008), and the Sumatran representa - DGR = dart gland retractor muscle; D = diameter; tives include H. abbasi Maassen, 2009, H. goliath E = epiphallus; EC = epiphallic caecum; F = fla - van Benthem Jutting, 1959, H. humphreysiana gellum; GA = genital atrium; H = height; ht = ho - (Lea, 1840), H. obliquata (Reeve, 1852) and H. lotype; P = penis; PRM = penial retractor muscle; obliqueundulata van Benthem Jutting, 1959 (see S = spermatheca; sd = standard deviation; U = um - van Benthem Jutting, 1959; Dharma, 2005; Maas - bilicus; V = vagina; VD = was deferens; x = mean sen, 2009). -

Download Article (PDF)
Rec. zool. Surv. India, 98(Part-3) : 67-70, 2000 NEW RECORDS OF PESTIFEROUS LAND MOLLUSCS FROM RAJASTHAN, INDIA SEEMA KUMAR and S.I. AHMED Arid forest Research Institute, P. O. Krishi Mandi, New Palsi Road, Jodhpur-342 005, Rajasthan, India INTRODUctION More than 1500 species of land mollusc are recorded from India. Out of these, twelve species viz. Achatina fulica Bowdich, Ariophanla bajadera (Pfeiffer), Ariophanta ligulata Ferrussac, Ariophanta solata (Benson), Bensonia monticola Hutton, Cryptozona belangiri Deshayes, Cryptozona (Nilgiria) semiru~ata (Seck.), Cryptozona (Xestina) bistrialis Beck., Macrochlamys indica (Godwin-Austen), Opeas gracile (Hutton), Zootecus insularis (Ehrenberg) and Mariaella dussumieri (Gray) are known to cause damage to agricultural, horticultural and plantation crops in India (Raut & Ghosh, 1984; Srivastava, 1992 and Subba Rao, 1975). So far, only two pestiferous land moliusc Opeas gracile (Hutton) and Zootecus insularis (Ehrenberg) are reported from Rajasthan (Subba Rao & Mitra, 1979 and Raut & Ghosh, 1984). Laevicaulis alte (Ferussac) is reported from Udaipur, Rajasthan but not as a pest (Ray & Mukherjee, 1963). The present paper reports for the first time, two more pestiferous land mollusc - Laevicaulis alte (Ferussac) and Macrochlamys indica (Godwin-Austen), as severe pests of neem seedlings in forest nurseries of Rajasthan along with new distributional records. SYSTEMATIC ACCOUNT 1. Laevicaulis aile (Ferussac, 1821) Phyllum MOLLUSCA Class GASTROPODA Subclass GYMNOMORPHA Order STYLOMMATOPHORA Family VERONICELLIDAE Genus Laevicaulis Simtoth, 1913. Laevicaulis aile (Ferussac, 1821) 1821. Vaginulus aile Ferussac, Tab1. Syst. Anim. Moll., Paris, p. 14. 1925. Meisenheimeria aile Hoffman. Tena. Z. Naturw., Jena, 61 : 226-228. PI. V, Fig. 45 b. 68 RECORDS OF THE ZOOLOGICAL SURVEY OF INDIA 1953. -

The Prevalence of Angiostrongylus Cantonensis/Mackerrasae Complex in Molluscs from the Sydney Region
RESEARCH ARTICLE The Prevalence of Angiostrongylus cantonensis/mackerrasae Complex in Molluscs from the Sydney Region Douglas Chan1,2*, Joel Barratt2,3, Tamalee Roberts1, Rogan Lee4, Michael Shea5, Deborah Marriott1, John Harkness1, Richard Malik6, Malcolm Jones7, Mahdis Aghazadeh7, John Ellis3, Damien Stark1 1 Department of Microbiology, SydPath, St. Vincent’s Hospital, Victoria St, Darlinghurst, NSW, Australia, 2 i3 Institute, University of Technology, Sydney, Ultimo, NSW, Australia, 3 School of Medical and Molecular Sciences, University of Technology, Sydney, Ultimo, NSW, Australia, 4 Centre for Infectious Diseases and Microbiology Laboratory Services, ICPMR, Westmead Hospital, Westmead, NSW, Australia, 5 Malacology Department, Australian Museum, Sydney, NSW, Australia, 6 Centre for Veterinary Education, University of Sydney, Camperdown, NSW, Australia, 7 School of Veterinary Science, The University of Queensland, a11111 Gatton, Queensland, Australia * [email protected] Abstract Angiostrongylus cantonensis and Angiostrongylus mackerrasae are metastrongyloid nema- OPEN ACCESS todes that infect various rat species. Terrestrial and aquatic molluscs are intermediate hosts Citation: Chan D, Barratt J, Roberts T, Lee R, Shea of these worms while humans and dogs are accidental hosts. Angiostrongylus cantonensis M, Marriott D, et al. (2015) The Prevalence of Angiostrongylus cantonensis/mackerrasae Complex is the major cause of angiostrongyliasis, a disease characterised by eosinophilic meningitis. in Molluscs from the Sydney Region. PLoS ONE Although both A. cantonensis and A. mackerrasae are found in Australia, A. cantonensis ap- 10(5): e0128128. doi:10.1371/journal.pone.0128128 pears to account for most infections in humans and animals. Due to the occurrence of sev- Academic Editor: Henk D. F. H. Schallig, Royal eral severe clinical cases in Sydney and Brisbane, the need for epidemiological studies on Tropical Institute, NETHERLANDS angiostrongyliasis in this region has become apparent. -

Veronicella Spp.*
Veronicella spp.* *In April 2013, the family Veronicellidae, a target on the 2013 and 2014 AHP Prioritized Pest Lists, was broken down into six genera of concern, including Veronicella spp. Information in the datasheet may be at the family, genus, or species level. Information for specific species within the genus is included when known and relevant; other species may occur in the genus and are still reportable at the genus level. Portions of this document were taken Figure 1. Veronicella cubensis (Pfeiffer), (Image directly from the New Pest Response courtesy of David Robinson, USDA-APHIS-PPQ) Guidelines for Tropical Terrestrial Gastropods (USDA-APHIS, 2010a). Scientific Names Veronicella cubensis (Pfeiffer, 1840) Veronicella sloanii (Cuvier, 1817) Synonyms: Veronicella cubensis Onchidium cubense Pfeiffer, 1840, Onchidium cubensis, Veronicella cubensis Thomé [Thomé], 1975 Veronicella sloanei Vaginulus sloanei Férussac, [Férussac] Vaginulus laevis de Blainville, 1817 Common Name No common name, leatherleaf slugs Figure 2. Veronicella sloanei (Cuvier), (Image courtesy of David Robinson, USDA-APHIS-PPQ) Veronicella cubensis: Cuban slug Veronicella sloanii: Pancake slug Type of Pest Mollusk Taxonomic Position Class: Gastropoda, Order: Systellommatophora, Family: Veronicellidae Last update: May 2014 1 Reason for Inclusion in Manual CAPS Target: AHP Prioritized Pest List for FY 2011 – 2015* *Originally listed under the family Veronicellidae. Pest Description Veronicellidae are anatomically distinct from many other terrestrial slugs in that they have a posterior anus, eyes on contractile tentacles, and no pulmonate lung. The sensory tentacles are bilobed. This family also lacks a mantel cavity (Runham and Hunter, 1970). Although this family is fairly easy to tell apart from others, species within this family can be difficult to distinguish due to similar morphology between species and multiple color variations within a single species. -

Angiostrongylus Cantonensis: a Review of Its Distribution, Molecular Biology and Clinical Significance As a Human
See discussions, stats, and author profiles for this publication at: https://www.researchgate.net/publication/303551798 Angiostrongylus cantonensis: A review of its distribution, molecular biology and clinical significance as a human... Article in Parasitology · May 2016 DOI: 10.1017/S0031182016000652 CITATIONS READS 4 360 10 authors, including: Indy Sandaradura Richard Malik Centre for Infectious Diseases and Microbiolo… University of Sydney 10 PUBLICATIONS 27 CITATIONS 522 PUBLICATIONS 6,546 CITATIONS SEE PROFILE SEE PROFILE Derek Spielman Rogan Lee University of Sydney The New South Wales Department of Health 34 PUBLICATIONS 892 CITATIONS 60 PUBLICATIONS 669 CITATIONS SEE PROFILE SEE PROFILE Some of the authors of this publication are also working on these related projects: Create new project "The protective rate of the feline immunodeficiency virus vaccine: An Australian field study" View project Comparison of three feline leukaemia virus (FeLV) point-of-care antigen test kits using blood and saliva View project All content following this page was uploaded by Indy Sandaradura on 30 May 2016. The user has requested enhancement of the downloaded file. All in-text references underlined in blue are added to the original document and are linked to publications on ResearchGate, letting you access and read them immediately. 1 Angiostrongylus cantonensis: a review of its distribution, molecular biology and clinical significance as a human pathogen JOEL BARRATT1,2*†, DOUGLAS CHAN1,2,3†, INDY SANDARADURA3,4, RICHARD MALIK5, DEREK SPIELMAN6,ROGANLEE7, DEBORAH MARRIOTT3, JOHN HARKNESS3, JOHN ELLIS2 and DAMIEN STARK3 1 i3 Institute, University of Technology Sydney, Ultimo, NSW, Australia 2 School of Life Sciences, University of Technology Sydney, Ultimo, NSW, Australia 3 Department of Microbiology, SydPath, St. -

Fauna of New Zealand Ko Te Aitanga Pepeke O Aotearoa
aua o ew eaa Ko te Aiaga eeke o Aoeaoa IEEAE SYSEMAICS AISOY GOU EESEAIES O ACAE ESEAC ema acae eseac ico Agicuue & Sciece Cee P O o 9 ico ew eaa K Cosy a M-C aiièe acae eseac Mou Ae eseac Cee iae ag 917 Aucka ew eaa EESEAIE O UIESIIES M Emeso eame o Eomoogy & Aima Ecoogy PO o ico Uiesiy ew eaa EESEAIE O MUSEUMS M ama aua Eiome eame Museum o ew eaa e aa ogaewa O o 7 Weigo ew eaa EESEAIE O OESEAS ISIUIOS awece CSIO iisio o Eomoogy GO o 17 Caea Ciy AC 1 Ausaia SEIES EIO AUA O EW EAA M C ua (ecease ue 199 acae eseac Mou Ae eseac Cee iae ag 917 Aucka ew eaa Fauna of New Zealand Ko te Aitanga Pepeke o Aotearoa Number / Nama 38 Naturalised terrestrial Stylommatophora (Mousca Gasooa Gay M ake acae eseac iae ag 317 amio ew eaa 4 Maaaki Whenua Ρ Ε S S ico Caeuy ew eaa 1999 Coyig © acae eseac ew eaa 1999 o a o is wok coee y coyig may e eouce o coie i ay om o y ay meas (gaic eecoic o mecaica icuig oocoyig ecoig aig iomaio eiea sysems o oewise wiou e wie emissio o e uise Caaoguig i uicaio AKE G Μ (Gay Micae 195— auase eesia Syommaooa (Mousca Gasooa / G Μ ake — ico Caeuy Maaaki Weua ess 1999 (aua o ew eaa ISS 111-533 ; o 3 IS -7-93-5 I ie 11 Seies UC 593(931 eae o uIicaio y e seies eio (a comee y eo Cosy usig comue-ase e ocessig ayou scaig a iig a acae eseac M Ae eseac Cee iae ag 917 Aucka ew eaa Māoi summay e y aco uaau Cosuas Weigo uise y Maaaki Weua ess acae eseac O o ico Caeuy Wesie //wwwmwessco/ ie y G i Weigo o coe eoceas eicuaum (ue a eigo oaa (owe (IIusao G M ake oucio o e coou Iaes was ue y e ew eaIa oey oa ue oeies eseac -

Slug: an Emerging Menace in Agriculture: a Review
Journal of Entomology and Zoology Studies 2020; 8(4): 01-06 E-ISSN: 2320-7078 P-ISSN: 2349-6800 www.entomoljournal.com Slug: An emerging menace in agriculture: A JEZS 2020; 8(4): 01-06 © 2020 JEZS review Received: 01-05-2020 Accepted: 03-06-2020 Partha Pratim Gyanudoy Das, Badal Bhattacharyya, Sudhansu Partha Pratim Gyanudoy Das All India Network Project on Bhagawati, Elangbam Bidyarani Devi, Nang Sena Manpoong and K Soil Arthropod Pests, Sindhura Bhairavi Department of Entomology, Assam Agricultural University, Jorhat, Assam, India Abstract Most of the terrestrial slugs are potential threat to agriculture across the globe. Their highly adaptive Badal Bhattacharyya nature helps them to survive in both temperate and tropical climates which is one of the major reasons of All India Network Project on its abundant species diversity. It is not only a severe problem in different seedlings of nursery and Soil Arthropod Pests, orchards, also a worry factor for the seeds of legumes sown in furrows. The whitish slimy mucus Department of Entomology, generated by this pest makes the flower and vegetables unfit for sale. However, despite of its euryphagic Assam Agricultural University, nature, very few works have been carried out on slug morphology, biology, ecology, taxonomy and its Jorhat, Assam, India management in India. This review article tries to integrate the information of economically important slug species of the world as well as India, their bio-ecology, nature of damage, favorable factors with Sudhansu Bhagawati special emphasis on eco-friendly management tactics of this particular gastropod pest. All India Network Project on Soil Arthropod Pests, Keywords: Slug, euryphagic, bio-ecology, management, gastropod pest Department of Entomology, Assam Agricultural University, Jorhat, Assam, India Introduction With a number of 80,000 to 135,000 members, mollusc ranks second largest invertebrate Elangbam Bidyarani Devi group in the world, out of which 1129 species of terrestrial molluscs are found in India [1, 2, 3]. -

A “Love” Dart Allohormone Identified in the Mucous Glands of Hermaphroditic Land Snails
crossmark THE JOURNAL OF BIOLOGICAL CHEMISTRY VOL. 291, NO. 15, pp. 7938–7950, April 8, 2016 © 2016 by The American Society for Biochemistry and Molecular Biology, Inc. Published in the U.S.A. A “Love” Dart Allohormone Identified in the Mucous Glands of Hermaphroditic Land Snails*□S Received for publication, November 22, 2015, and in revised form, January 14, 2016 Published, JBC Papers in Press, January 27, 2016, DOI 10.1074/jbc.M115.704395 Michael J. Stewart‡, Tianfang Wang‡, Joris M. Koene§, Kenneth B. Storey¶, and Scott F. Cummins‡1 From the ‡Genecology Research Centre, Faculty of Science, Health, Education and Engineering, University of the Sunshine Coast, Maroochydore, Queensland 4558, Australia , the §Department of Ecological Science, Faculty of Earth and Life Sciences, Vrije Universiteit, 1081HV Amsterdam, The Netherlands, and the ¶Institute of Biochemistry and Department of Biology, Carleton University, Ottawa, Ontario K1S 5B6, Canada Animals have evolved many ways to enhance their own repro- tion, at the level of the sperm, and this process seems to have ductive success. One bizarre sexual ritual is the “love” dart become an especially important evolutionary driving force shooting of helicid snails, which has courted many theories among a group of species with a different reproductive strategy: regarding its precise function. Acting as a hypodermic needle, simultaneous hermaphrodites that do not self-fertilize (4–6). the dart transfers an allohormone that increases paternity suc- Helicid land snail copulation lasts 2–6 h and includes the Downloaded from cess. Its precise physiological mechanism of action within the unique use of calcareous (calcium carbonate) “love” darts that recipient snail is to close off the entrance to the sperm digestion are pierced through the body wall of the mating partner during organ via a contraction of the copulatory canal, thereby delaying courtship (7–10). -

Gastropoda: Stylommatophora)1 John L
EENY-494 Terrestrial Slugs of Florida (Gastropoda: Stylommatophora)1 John L. Capinera2 Introduction Florida has only a few terrestrial slug species that are native (indigenous), but some non-native (nonindigenous) species have successfully established here. Many interceptions of slugs are made by quarantine inspectors (Robinson 1999), including species not yet found in the United States or restricted to areas of North America other than Florida. In addition to the many potential invasive slugs originating in temperate climates such as Europe, the traditional source of invasive molluscs for the US, Florida is also quite susceptible to invasion by slugs from warmer climates. Indeed, most of the invaders that have established here are warm-weather or tropical species. Following is a discus- sion of the situation in Florida, including problems with Figure 1. Lateral view of slug showing the breathing pore (pneumostome) open. When closed, the pore can be difficult to locate. slug identification and taxonomy, as well as the behavior, Note that there are two pairs of tentacles, with the larger, upper pair ecology, and management of slugs. bearing visual organs. Credits: Lyle J. Buss, UF/IFAS Biology as nocturnal activity and dwelling mostly in sheltered Slugs are snails without a visible shell (some have an environments. Slugs also reduce water loss by opening their internal shell and a few have a greatly reduced external breathing pore (pneumostome) only periodically instead of shell). The slug life-form (with a reduced or invisible shell) having it open continuously. Slugs produce mucus (slime), has evolved a number of times in different snail families, which allows them to adhere to the substrate and provides but this shell-free body form has imparted similar behavior some protection against abrasion, but some mucus also and physiology in all species of slugs. -

In Vitro Production and Biocontrol Potential of Nematodes Associated with Molluscs
In vitro production and biocontrol potential of nematodes associated with molluscs by Annika Pieterse Dissertation presented for the degree of Doctor of Nematology in the Faculty of AgriSciences at Stellenbosch University Co-supervisor: Professor Antoinette Paula Malan Co-supervisor: Doctor Jenna Louise Ross March 2020 Stellenbosch University https://scholar.sun.ac.za Declaration By submitting this thesis electronically, I declare that the entirety of the work contained therein is my own, original work, that I am the sole author thereof (save to the extent explicitly otherwise stated), that reproduction and publication thereof by Stellenbosch University will not infringe any third party rights and that I have not previously in its entirety or in part submitted it for obtaining any qualification. This dissertation includes one original paper published in a peer-reviewed journal. The development and writing of the paper was the principal responsibility of myself and, for each of the cases where this is not the case, a declaration is included in the dissertation indicating the nature and extent of the contributions of co-authors. March 2020 Copyright © 2020 Stellenbosch University All rights reserved II Stellenbosch University https://scholar.sun.ac.za Acknowledgements First and foremost, I would like to thank my two supervisors, Prof Antoinette Malan and Dr Jenna Ross. This thesis would not have been possible without their help, patience and expertise. I am grateful for the opportunity to have been part of this novel work in South Africa. I would like to thank Prof. Des Conlong for welcoming me at SASRI in KwaZulu-Natal and organizing slug collections with local growers, as well as Sheila Storey for helping me transport the slugs from KZN. -
Sarasinula Spp.*
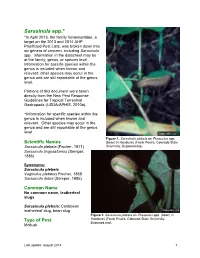
Sarasinula spp.* *In April 2013, the family Veronicellidae, a target on the 2013 and 2014 AHP Prioritized Pest Lists, was broken down into six genera of concern, including Sarasinula spp. Information in the datasheet may be at the family, genus, or species level. Information for specific species within the genus is included when known and relevant; other species may occur in the genus and are still reportable at the genus level. Portions of this document were taken directly from the New Pest Response Guidelines for Tropical Terrestrial Gastropods (USDA-APHIS, 2010a). *Information for specific species within the genus is included when known and relevant. Other species may occur in the genus and are still reportable at the genus level. Figure 1. Sarasinula plebeia on Phaseolus spp. Scientific Names (bean) in Honduras (Frank Peairs, Colorado State Sarasinula plebeia (Fischer, 1871) University, Bugwood.org). Sarasinula linguaeformis (Semper, 1885) Synonyms: Sarasinula plebeia Vaginulus plebeius Fischer, 1868 Sarasinula dubia (Semper, 1885) Common Name No common name, leatherleaf slugs Sarasinula plebeia: Caribbean leatherleaf slug, bean slug Figure 2. Sarasinula plebeia on Phaseolus spp. (bean) in Honduras (Frank Peairs, Colorado State University, Type of Pest Bugwood.org). Mollusk Last update: August 2014 1 Taxonomic Position Class: Gastropoda, Order: Systellommatophora, Family: Veronicellidae Reason for Inclusion in Manual CAPS Target: AHP Prioritized Pest List for FY 2011 – 2015* *Originally listed under the family Veronicellidae. Pest Description Veronicellidae are anatomically distinct from many other terrestrial slugs in that they have a posterior anus, eyes on contractile tentacles, and no pulmonate lung. The sensory tentacles are bilobed. This family also lacks a mantel cavity (Runham and Hunter, 1970).