Atmospheric Pressure ×
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Fog and Low Clouds As Troublemakers During Wildfi Res
When Our Heads Are in the Clouds Sometimes water droplets do not freeze in below- Detecting fog from space Up to 60,000 ft (18,000m) freezing temperatures. This happens if they do not have Weather satellites operated by the National Oceanic The fog comes a surface (like a dust particle or an ice crystal) upon and Atmospheric Administration (NOAA) collect data on on little cat feet. which to freeze. This below-freezing liquid water becomes clouds and storms. Cirrus Commercial Jetliner “supercooled.” Then when it touches a surface whose It sits looking (36,000 ft / 11,000m) temperature is below freezing, such as a road or sidewalk, NOAA operates two different types of satellites. over harbor and city Geostationary satellites orbit at about 22,236 miles Breitling Orbiter 3 the water will freeze instantly, making a super-slick icy on silent haunches (34,000 ft / 10,400m) Cirrocumulus coating on whatever it touches. This condition is called (35,786 kilometers) above sea level at the equator. At this and then moves on. Mount Everest (29,035 ft / 8,850m) freezing fog. altitude, the satellite makes one Earth orbit per day, just Carl Sandburg Cirrostratus as Earth rotates once per day. Thus, the satellite seems to 20,000 feet (6,000 m) Cumulonimbus hover over one spot below and keeps its “birds’-eye view” of nearly half the Earth at once. Altocumulus The other type of NOAA satellites are polar satellites. Their orbits pass over, or nearly over, the North and South Clear and cloudy regions over the U.S. -

Pressure, Its Units of Measure and Pressure References
_______________ White Paper Pressure, Its Units of Measure and Pressure References Viatran Phone: 1‐716‐629‐3800 3829 Forest Parkway Fax: 1‐716‐693‐9162 Suite 500 [email protected] Wheatfield, NY 14120 www.viatran.com This technical note is a summary reference on the nature of pressure, some common units of measure and pressure references. Read this and you won’t have to wait for the movie! PRESSURE Gas and liquid molecules are in constant, random motion called “Brownian” motion. The average speed of these molecules increases with increasing temperature. When a gas or liquid molecule collides with a surface, momentum is imparted into the surface. If the molecule is heavy or moving fast, more momentum is imparted. All of the collisions that occur over a given area combine to result in a force. The force per unit area defines the pressure of the gas or liquid. If we add more gas or liquid to a constant volume, then the number of collisions must increase, and therefore pressure must increase. If the gas inside the chamber is heated, the gas molecules will speed up, impact with more momentum and pressure increases. Pressure and temperature therefore are related (see table at right). The lowest pressure possible in nature occurs when there are no molecules at all. At this point, no collisions exist. This condition is known as a pure vacuum, or the absence of all matter. It is also possible to cool a liquid or gas until all molecular motion ceases. This extremely cold temperature is called “absolute zero”, which is -459.4° F. -
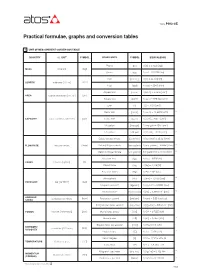
Practical Formulae, Graphs and Conversion Tables
Table P003-4/E Practical formulae, graphs and conversion tables 1 UNIT OF MEASUREMENT CONVERSION TABLE QUANTITY S.I. UNIT SYMBOL OTHER UNITS SYMBOL EQUIVALENCE Pound [lb] 1 [lb] = 0,4536 [kg] kilogram [kg] MASS Ounce [oz] 1 [oz] = 0,02335 [kg] Inch [in] or [”] 1 [in] = 25,40 [mm] millimeter [10-3 m] [mm] LENGTH Foot [foot] 1 [foot] = 304,8 [mm] Square inch [sq in] 1 [sq in] = 6,4516 [cm2] -4 2 [cm2] AREA square centimeter [10 m ] Square foot [sq ft] 1 [sq ft] = 929,034 [cm2] Liter [l] 1 [l] = 1000 [cm3] Cubic inch [cu in] 1 [cu in] = 16,3870 [cm3] * cubic centimeter [10-6 m3] [cm3] Cubic foot [cu ft] 1 [cu ft] = 28317 [cm3] CAPACITY UK gallon [Imp gal] 1 [Imp gal] = 4546 [cm3] US gallon [US gal] 1 [US gal] = 3785 [cm3] * Cubic foot per minute [cu ft/min] 1 [cu ft/min] = 28,32 [l/min] liter per minute [l/min] Gallon (UK) per minute [Imp gal/min] 1 [Imp gal/min] = 4,5456 [l/min] * FLOW RATE Gallon (US) per minute [US gal/min] [US gal/min] = 3,7848 [l/min] * Kilogram force [kgf] 1 [kgf] = 9,806 [N] Newton [kgm/s2] [N] FORCE Pound force [lbf] 1 [lbf] = 4,448 [N] Pascal [1 N/m2] [Pa] 1 [Pa] = 10-5 [bar] Atmosphere [atm] 1 [atm] = 1,0132 [bar] * bar [105 N/m2] [bar] PRESSURE 2 2 2 Kilogram force/cm [kgf/cm ] 1 [kgf/cm ] = 0,9806 [bar] 2 2 -2 Pound force/in [lbf /in ] or [psi] 1 [psi] = 6,8948•10 [bar] * ANGULAR revolution per minute [rpm] Radian per second [rad/sec] 1 [rpm] = 9,55 [rad/sec] SPEED -3 Kilogram per meter second [kgf •m/s] 1 [kgf •m/s] = 9,803•10 [kW] kilowatt [1000 Nm/s] [kW] Metric horse power [CV] 1 [CV] = 0,7355 [kW] POWER -

American and BRITISH UNITS of Measurement to SI UNITS
AMERICAN AND BRITISH UNITS OF MEASUREMENT TO SI UNITS UNIT & ABBREVIATION SI UNITS CONVERSION* UNIT & ABBREVIATION SI UNITS CONVERSION* UNITS OF LENGTH UNITS OF MASS 1 inch = 40 lines in 2.54 cm 0.393701 1 grain gr 64.7989 mg 0.0154324 1 mil 25.4 µm 0.03937 1 dram dr 1.77185 g 0.564383 1 line 0.635 mm 1.57480 1 ounce = 16 drams oz 28.3495 g 0.0352739 1 foot = 12 in = 3 hands ft 30.48 cm 0.0328084 1 pound = 16 oz lb 0.453592 kg 2.204622 1 yard = 3 feet = 4 spans yd 0.9144 m 1.09361 1 quarter = 28 lb 12.7006 kg 0.078737 1 fathom = 2 yd fath 1.8288 m 0.546807 1 hundredweight = 112 lb cwt 50.8024 kg 0.0196841 1 rod (perch, pole) rd 5.0292 m 0.198839 1 long hundredweight l cwt 50.8024 kg 0.0196841 1 chain = 100 links ch 20.1168 m 0.0497097 1 short hundredweight sh cwt 45.3592 kg 0.0220462 1 furlong = 220 yd fur 0.201168 km 4.97097 1 ton = 1 long ton tn, l tn 1.016047 t 0.984206 1 mile (Land Mile) mi 1.60934 km 0.62137 1 short ton = 2000 lb sh tn 0.907185 t 1.102311 1 nautical mile (intl.) n mi, NM 1.852 km 0.539957 1 knot (Knoten) kn 1.852 km/h 0.539957 UNITS OF FORCE 1 pound-weight lb wt 4.448221 N 0.2248089 UNITS OF AREA 1 pound-force LB, lbf 4.448221 N 0.2248089 1 square inch sq in 6.4516 cm2 0.155000 1 poundal pdl 0.138255 N 7.23301 1 circular inch 5.0671 cm2 0.197352 1 kilogram-force kgf, kgp 9.80665 N 0.1019716 1 square foot = 144 sq in sq ft 929.03 cm2 1.0764 x 10-4 1 short ton-weight sh tn wt 8.896444 kN 0.1124045 1 square yard = 9 sq ft sq yd 0.83613 m2 1.19599 1 long ton-weight l tn wt 9.964015 kN 0.1003611 1 acre = 4 roods 4046.8 -

Aviation Glossary
AVIATION GLOSSARY 100-hour inspection – A complete inspection of an aircraft operated for hire required after every 100 hours of operation. It is identical to an annual inspection but may be performed by any certified Airframe and Powerplant mechanic. Absolute altitude – The vertical distance of an aircraft above the terrain. AD - See Airworthiness Directive. ADC – See Air Data Computer. ADF - See Automatic Direction Finder. Adverse yaw - A flight condition in which the nose of an aircraft tends to turn away from the intended direction of turn. Aeronautical Information Manual (AIM) – A primary FAA publication whose purpose is to instruct airmen about operating in the National Airspace System of the U.S. A/FD – See Airport/Facility Directory. AHRS – See Attitude Heading Reference System. Ailerons – A primary flight control surface mounted on the trailing edge of an airplane wing, near the tip. AIM – See Aeronautical Information Manual. Air data computer (ADC) – The system that receives and processes pitot pressure, static pressure, and temperature to present precise information in the cockpit such as altitude, indicated airspeed, true airspeed, vertical speed, wind direction and velocity, and air temperature. Airfoil – Any surface designed to obtain a useful reaction, or lift, from air passing over it. Airmen’s Meteorological Information (AIRMET) - Issued to advise pilots of significant weather, but describes conditions with lower intensities than SIGMETs. AIRMET – See Airmen’s Meteorological Information. Airport/Facility Directory (A/FD) – An FAA publication containing information on all airports, seaplane bases and heliports open to the public as well as communications data, navigational facilities and some procedures and special notices. -

Solar Energy Generation Model for High Altitude Long Endurance Platforms
Solar Energy Generation Model for High Altitude Long Endurance Platforms Mathilde Brizon∗ KTH - Royal Institute of Technology, Stockholm, Sweden For designing and evaluating new concepts for HALE platforms, the energy provided by solar cells is a key factor. The purpose of this thesis is to model the electrical power which can be harnessed by such a platform along any flight trajectory for different aircraft designs. At first, a model of the solar irradiance received at high altitude will be performed using the solar irradiance models already existing for ground level applications as a basis. A calculation of the efficiency of the energy generation will be performed taking into account each solar panel's position as well as shadows casted by the aircraft's structure. The evaluated set of trajectories allows a stationary positioning of a hale platform with varying wind conditions, time of day and latitude for an exemplary aircraft configuration. The qualitative effects of specific parameter changes on the harnessed solar energy is discussed as well as the fidelity of the energy generation model results. Nomenclature δ Solar declination ({) EQE Quantum efficiency (%) η Efficiency (%) hg Altitude of the aircraft (m) ◦ Γ Day angle ( ) hO3 Height of max ozone concentration(m) ◦ −2 −1 λg Longitude aircraft ( ) Id Direct irradiance (W:m .µm ) ◦ −2 −1 ! Hour angle ( ) Is Diffuse irradiance (W:m .µm ) ◦ −2 −1 φg Latitude of the aircraft ( ) Itot Total irradiance (W:m .µm ) ◦ −1 ◦ τ Rayleigh optical depth ({) kPmax;T Temperature Coefficient (%: C ) 2 A Solar cell -

1 the Atmosphere of Pluto As Observed by New Horizons G
The Atmosphere of Pluto as Observed by New Horizons G. Randall Gladstone,1,2* S. Alan Stern,3 Kimberly Ennico,4 Catherine B. Olkin,3 Harold A. Weaver,5 Leslie A. Young,3 Michael E. Summers,6 Darrell F. Strobel,7 David P. Hinson,8 Joshua A. Kammer,3 Alex H. Parker,3 Andrew J. Steffl,3 Ivan R. Linscott,9 Joel Wm. Parker,3 Andrew F. Cheng,5 David C. Slater,1† Maarten H. Versteeg,1 Thomas K. Greathouse,1 Kurt D. Retherford,1,2 Henry Throop,7 Nathaniel J. Cunningham,10 William W. Woods,9 Kelsi N. Singer,3 Constantine C. C. Tsang,3 Rebecca Schindhelm,3 Carey M. Lisse,5 Michael L. Wong,11 Yuk L. Yung,11 Xun Zhu,5 Werner Curdt,12 Panayotis Lavvas,13 Eliot F. Young,3 G. Leonard Tyler,9 and the New Horizons Science Team 1Southwest Research Institute, San Antonio, TX 78238, USA 2University of Texas at San Antonio, San Antonio, TX 78249, USA 3Southwest Research Institute, Boulder, CO 80302, USA 4National Aeronautics and Space Administration, Ames Research Center, Space Science Division, Moffett Field, CA 94035, USA 5The Johns Hopkins University Applied Physics Laboratory, Laurel, MD 20723, USA 6George Mason University, Fairfax, VA 22030, USA 7The Johns Hopkins University, Baltimore, MD 21218, USA 8Search for Extraterrestrial Intelligence Institute, Mountain View, CA 94043, USA 9Stanford University, Stanford, CA 94305, USA 10Nebraska Wesleyan University, Lincoln, NE 68504 11California Institute of Technology, Pasadena, CA 91125, USA 12Max-Planck-Institut für Sonnensystemforschung, 37191 Katlenburg-Lindau, Germany 13Groupe de Spectroscopie Moléculaire et Atmosphérique, Université Reims Champagne-Ardenne, 51687 Reims, France *To whom correspondence should be addressed. -

Guide for the Use of the International System of Units (SI)
Guide for the Use of the International System of Units (SI) m kg s cd SI mol K A NIST Special Publication 811 2008 Edition Ambler Thompson and Barry N. Taylor NIST Special Publication 811 2008 Edition Guide for the Use of the International System of Units (SI) Ambler Thompson Technology Services and Barry N. Taylor Physics Laboratory National Institute of Standards and Technology Gaithersburg, MD 20899 (Supersedes NIST Special Publication 811, 1995 Edition, April 1995) March 2008 U.S. Department of Commerce Carlos M. Gutierrez, Secretary National Institute of Standards and Technology James M. Turner, Acting Director National Institute of Standards and Technology Special Publication 811, 2008 Edition (Supersedes NIST Special Publication 811, April 1995 Edition) Natl. Inst. Stand. Technol. Spec. Publ. 811, 2008 Ed., 85 pages (March 2008; 2nd printing November 2008) CODEN: NSPUE3 Note on 2nd printing: This 2nd printing dated November 2008 of NIST SP811 corrects a number of minor typographical errors present in the 1st printing dated March 2008. Guide for the Use of the International System of Units (SI) Preface The International System of Units, universally abbreviated SI (from the French Le Système International d’Unités), is the modern metric system of measurement. Long the dominant measurement system used in science, the SI is becoming the dominant measurement system used in international commerce. The Omnibus Trade and Competitiveness Act of August 1988 [Public Law (PL) 100-418] changed the name of the National Bureau of Standards (NBS) to the National Institute of Standards and Technology (NIST) and gave to NIST the added task of helping U.S. -

Pressure Measurement Explained
Pressure measurement explained Rev A1, May 25th, 2018 Sens4Knowledge Sens4 A/S – Nordre Strandvej 119 G – 3150 Hellebaek – Denmark Phone: +45 8844 7044 – Email: [email protected] www.sens4.com Sens4Knowledge Pressure measurement explained Introduction Pressure is defined as the force per area that can be exerted by a liquid, gas or vapor etc. on a given surface. The applied pressure can be measured as absolute, gauge or differential pressure. Pressure can be measured directly by measurement of the applied force or indirectly, e.g. by the measurement of the gas properties. Examples of indirect measurement techniques that are using gas properties are thermal conductivity or ionization of gas molecules. Before mechanical manometers and electronic diaphragm pressure sensors were invented, pressure was measured by liquid manometers with mercury or water. Pressure standards In physical science the symbol for pressure is p and the SI (abbreviation from French Le Système. International d'Unités) unit for measuring pressure is pascal (symbol: Pa). One pascal is the force of one Newton per square meter acting perpendicular on a surface. Other commonly used pressure units for stating the pressure level are psi (pounds per square inch), torr and bar. Use of pressure units have regional and applicational preference: psi is commonly used in the United States, while bar the preferred unit of measure in Europe. In the industrial vacuum community, the preferred pressure unit is torr in the United States, mbar in Europe and pascal in Asia. Unit conversion Pa bar psi torr atm 1 Pa = 1 1×10-5 1.45038×10-4 7.50062×10-3 9.86923×10-6 1 bar = 100,000 1 14.5038 750.062 0.986923 1 psi = 6,894.76 6.89476×10-2 1 51.7149 6.80460×10-2 1 torr = 133.322 1.33322×10-3 1.933768×10-2 1 1.31579×10-3 1 atm (standard) = 1013.25 1.01325 14.6959 760.000 1 According to the International Organization for Standardization the standard ISO 2533:1975 defines the standard atmospheric pressure of 101,325 Pa (1 atm, 1013.25 mbar or 14.6959 psi). -

Unit 11 Sound Speed of Sound Speed of Sound Sound Can Travel Through Any Kind of Matter, but Not Through a Vacuum
Unit 11 Sound Speed of Sound Speed of Sound Sound can travel through any kind of matter, but not through a vacuum. The speed of sound is different in different materials; in general, it is slowest in gases, faster in liquids, and fastest in solids. The speed depends somewhat on temperature, especially for gases. vair = 331.0 + 0.60T T is the temperature in degrees Celsius Example 1: Find the speed of a sound wave in air at a temperature of 20 degrees Celsius. v = 331 + (0.60) (20) v = 331 m/s + 12.0 m/s v = 343 m/s Using Wave Speed to Determine Distances At normal atmospheric pressure and a temperature of 20 degrees Celsius, speed of sound: v = 343m / s = 3.43102 m / s Speed of sound 750 mi/h Speed of light 670 616 629 mi/h c = 300,000,000m / s = 3.00 108 m / s Delay between the thunder and lightning Example 2: The thunder is heard 3 seconds after the lightning seen. Find the distance to storm location. The speed of sound is 345 m/s. distance = v t = (345m/s)(3s) = 1035m Example 3: Another phenomenon related to the perception of time delays between two events is an echo. In a canyon, an echo is heard 1.40 seconds after making the holler. Find the distance to the canyon wall (v=345m/s) distanceround trip = vt = (345 m/s )( 1.40 s) = 483 m d= 484/2=242m Applications: Sonar, Ultrasound, and Medical Imaging • Ultrasound or ultrasonography is a medical imaging technique that uses high frequency sound waves and their echoes. -

Common Units
Civil Engineering Hydraulics Pressure and Fluid Statics Leonard: It wouldn't kill us to meet new people. Sheldon: For the record, it could kill us to meet new people. Common Units ¢ In order to be able to discuss and analyze fluid problems we need to be able to understand some fundamental terms commonly used 2 Pressure 1 Common Units ¢ The most used term in hydraulics and fluid mechanics is probably pressure ¢ Pressure is defined as the normal force exerted by a fluid per unit of area l The important part of that definition is the normal (perpendicular) to the unit of area 3 Pressure Common Units ¢ The Pascal is a very small unit of pressure so it is most often encountered with a prefix to allow the numerical values to be easy to display ¢ Common prefixes are the Kilopascal (kPa=103Pa), the Megapascal (MPa=106Pa), and sometimes the Gigapascal (GPa=109Pa) 4 Pressure 2 Common Units ¢ A bar is defined as 105 Pa so a millibar (mbar) is defined as 10-3 bar so the millibar is 102 Pa The word bar finds its origin in the Greek word báros, meaning weight. 5 Pressure Common Units ¢ Standard atmospheric pressure or "the standard atmosphere" (1 atm) is defined as 101.325 kilopascals (kPa). 6 Pressure 3 Common Units ¢ This "standard pressure" is a purely arbitrary representative value for pressure at sea level, and real atmospheric pressures vary from place to place and moment to moment everywhere in the world. 7 Pressure Common Units ¢ Pressure is usually given in reference to some datum l Absolute pressure is given in reference to a system with -

Atmospheric Cold Front-Generated Waves in the Coastal Louisiana
Journal of Marine Science and Engineering Article Atmospheric Cold Front-Generated Waves in the Coastal Louisiana Yuhan Cao 1 , Chunyan Li 2,* and Changming Dong 1,3,* 1 School of Marine Sciences, Nanjing University of Information Science and Technology, Nanjing 210044, China; [email protected] 2 Department of Oceanography and Coastal Sciences, College of the Coast and Environment, Louisiana State University, Baton Rouge, LA 70803, USA 3 Southern Laboratory of Ocean Science and Engineering (Zhuhai), Zhuhai 519000, China * Correspondence: [email protected] (C.L.); [email protected] (C.D.); Tel.: +1-225-578-2520 (C.L.); +86-025-58695733 (C.D.) Received: 15 October 2020; Accepted: 9 November 2020; Published: 11 November 2020 Abstract: Atmospheric cold front-generated waves play an important role in the air–sea interaction and coastal water and sediment transports. In-situ observations from two offshore stations are used to investigate variations of directional waves in the coastal Louisiana. Hourly time series of significant wave height and peak wave period are examined for data from 2004, except for the summer time between May and August, when cold fronts are infrequent and weak. The intra-seasonal scale variations in the wavefield are significantly affected by the atmospheric cold frontal events. The wave fields and directional wave spectra induced by four selected cold front passages over the coastal Louisiana are discussed. It is found that significant wave height generated by cold fronts coming from the west change more quickly than that by other passing cold fronts. The peak wave direction rotates clockwise during the cold front events.