Biotechnological Approaches to Improve Potato: Review Article
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

International Union for the Protection of New Varieties of Plants Geneva
E TG/23/6 ORIGINAL: English DATE: 2004-03-31 INTERNATIONAL UNION FOR THE PROTECTION OF NEW VARIETIES OF PLANTS GENEVA * POTATO (Solanum tuberosum L.) GUIDELINES FOR THE CONDUCT OF TESTS FOR DISTINCTNESS, UNIFORMITY AND STABILITY Alternative Names: * Latin English French German Spanish Solanum tuberosum L., Potato Pomme de terre Kartoffel Papa, Patata S. tuberosum L. sensu lato ASSOCIATED DOCUMENTS These guidelines should be read in conjunction with document TG/1/3, “G eneral Introduction to the Examination of Distinctness, Uniformity and Stability and the Development of Harmonized Descriptions of New Varieties of Plants” (hereinafter referred to as the “General Introduction”) and its associated “TGP” documents. * These names were correct at the time of the introduction of these Test Guidelines but may be revised or updated. [Readers are advised to consult the UPOV Code, which can be found on the UPOV Website (www.upov.int), for the latest infor mation.] TG/23/6 Potato, 2004 -03 -31 - 2 - TABLE OF CONTENTS 1. SUBJECT OF THESE TES T GUIDELINES ................................ ................................ ................................ .. 3 2. MATERIAL REQUIRED ................................ ................................ ................................ ............................... 3 3. METHOD OF EXAMINATIO N................................ ................................ ................................ ..................... 3 3.1 Duration of Tests ................................ ................................ ............................... -

Best Practice Document for the Coexistence of Genetically Modified
JRC SCIENCE FOR POLICY REPORT European Coexistence Bureau (ECoB) Best practice document for the coexistence of genetically modified potato with conventional and organic farming Ivelin Rizov, Gerhard Rühl, Maren Langhof, Jonas Kathage, Emilio Rodríguez-Cerezo 2018 EUR 29047 EN This publication is a Science for Policy report by the Joint Research Centre (JRC), the European Commission’s science and knowledge service. It aims to provide evidence-based scientific support to the European policymaking process. The scientific output expressed does not imply a policy position of the European Commission. Neither the European Commission nor any person acting on behalf of the Commission is responsible for the use that might be made of this publication. Contact information Address: Edificio Expo. c/ Inca Garcilaso, 3. E-41092 Seville (Spain) Email: [email protected] Tel.: +34 954 48 8318 JRC Science Hub: https://ec.europa.eu/jrc JRC109645 EUR 29047 EN PDF ISBN 978-92-79-77694-6 ISSN 1831-9424 doi:10.2760/055172 Print ISBN 978-92-79-77695-3 ISSN 1018-5593 doi:10.2760/336072 Luxembourg: Publications Office of the European Union, 2018 © European Union, 2018 Reuse is authorised provided the source is acknowledged. The reuse policy of European Commission documents is regulated by Decision 2011/833/EU (OJ L 330, 14.12.2011, p. 39). For any use or reproduction of photos or other material that is not under the EU copyright, permission must be sought directly from the copyright holders. How to cite this report: Ivelin Rizov, Gerhard Rühl, Maren Langhof, Jonas Kathage, and Emilio Rodríguez-Cerezo, Best practice document for the coexistence of genetically modified potato with conventional and organic farming, EUR 29047 EN, Publications Office of the European Union, Luxembourg, 2018, ISBN 978-92-79-77694-6, doi:10.2760/055172, JRC109645. -

Potato - Wikipedia, the Free Encyclopedia
Potato - Wikipedia, the free encyclopedia Log in / create account Article Talk Read View source View history Our updated Terms of Use will become effective on May 25, 2012. Find out more. Main page Potato Contents From Wikipedia, the free encyclopedia Featured content Current events "Irish potato" redirects here. For the confectionery, see Irish potato candy. Random article For other uses, see Potato (disambiguation). Donate to Wikipedia The potato is a starchy, tuberous crop from the perennial Solanum tuberosum Interaction of the Solanaceae family (also known as the nightshades). The word potato may Potato Help refer to the plant itself as well as the edible tuber. In the region of the Andes, About Wikipedia there are some other closely related cultivated potato species. Potatoes were Community portal first introduced outside the Andes region four centuries ago, and have become Recent changes an integral part of much of the world's cuisine. It is the world's fourth-largest Contact Wikipedia food crop, following rice, wheat and maize.[1] Long-term storage of potatoes Toolbox requires specialised care in cold warehouses.[2] Print/export Wild potato species occur throughout the Americas, from the United States to [3] Uruguay. The potato was originally believed to have been domesticated Potato cultivars appear in a huge variety of [4] Languages independently in multiple locations, but later genetic testing of the wide variety colors, shapes, and sizes Afrikaans of cultivars and wild species proved a single origin for potatoes in the area -

Effect of Organic Potato Farming on Human and Environmental Health and Benefits from New Plant Breeding Techniques
sustainability Review Effect of Organic Potato Farming on Human and Environmental Health and Benefits from New Plant Breeding Techniques. Is It Only a Matter of Public Acceptance? Daniela Pacifico * and Roberta Paris Council for Agricultural Research and Economics Analysis (CREA), Centre for Research on Industrial Crops, Via di Corticella, 133, 40128 Bologna, Italy; [email protected] * Correspondence: daniela.pacifi[email protected]; Tel.: +39-051-631-6811 Academic Editor: Gerhart U. Ryffel Received: 26 July 2016; Accepted: 13 October 2016; Published: 20 October 2016 Abstract: Organic farming practices are commonly thought to reduce the environmental impact of agriculture and to preserve the naturalness of the products. Herein, we report the effect of crop management practices on nutritional and toxicological value of potato tubers. Comparative studies are often controversial and the results are dependent on genotype and methodological approach. Targeted analysis and “omics” strategies are discussed, pointing at the nutritional aspects and the corresponding biological and molecular processes involved. Organic farming supporters still do not accept the use of genetic modification to produce new varieties suited for organic agriculture and crop improvement by genetic engineering still sparks hot debate among various scientific and social factions whose major concern is the possible existence of unintended effects both on human and world health. In this context, the advent of “new plant breeding techniques” has reignited the discussion on genetic engineering and on the compatibility of the new technologies with an eco-friendly agriculture. Could cisgenic and genome-edited potatoes be new good options for organic agriculture? We discuss how these approaches can be used to address food security challenges and to overcome specific problems based on the biological characteristics of potato tubers, producing new varieties that can improve farmers’ profit with a lower impact on public opinion. -

Minnesota Area Ii Potato Research and Promotion Council and Northern
MINNESOTA AREA II POTATO RESEARCH AND PROMOTION COUNCIL AND NORTHERN PLAINS POTATO GROWERS ASSOCIATION 2019 RESEARCH REPORTS Table of Contents 3. Impact of Sublethal Dicamba & Glyphosate Rates on Three Chipping Potato Cultivars M. Brooke, H. Hatterman-Valenti, A. Robinson, G. Secor & A. Auwarter 7. Vine Desiccation as an Effective Disease Management Strategy to Control Verticillium Wilt of Potato N. Gudmestad 13. Nitrogen Fertilization Rate and Cold-induced Sweetening in Potato Tubers During Storage S. Gupta & C. Rosen 21. Pressure Flattening and Bruise Susceptibility Among New Fresh Market and Chip Varieties D. Haagenson 26. Adjuvent Comparison with Potato Desiccants, Grand Forks, 1 H. Hatterman-Valenti & C. Auwarter 27. Adjuvent Comparison with Potato Desiccants, Grand Forks, 2 H. Hatterman-Valenti and C. Auwarter 28. Evaluating SOP vs. MOP Programs in Russet Burbank Potato H. Hatterman-Valenti & C. Auwarter 29. Evaluating Single and Repeat Hail Event in “Clearwater” Potato H. Hatterman-Valenti & C. Auwarter 32. Baseline Evaluation of Pollinator Landscape Plantings Bordering Commercial Potato I. MacRae 36. Management of Colorado Potato Beetle in Minnesota & North Dakota I. MacRae 41. Managing PVY Vectors, 2018 I. MacRae 49. Carryover of Herbicides in Potato Production Systems A. Robinson, E. Brandvik, & P. Ihry 54. Effects of Planting Configuration & Plant Population Density on the N Response of Russet Burbank Tuber Yield & Size C. Rosen, J. Crants, M. McNearney, K. Olander& H. Barrett 66. Evaluation of Aspire, Micro-Essentials S10, & MicroEssentials SZ as Sources of Potassium, Phosphate, Sulfur, Boron & Zine for Russet Burbank Potatoes C. Rosen, J. Crants & M. McNearney 73. Evaluation of New Controlled Release Urea Fertilizer Products as N Sources for Russet Burbank Potatoes C. -

Common Scab Susceptibility of 24 Most Popular Potato Cultivars in USA, Utilizing a Greenhouse Assay with Three Different Pathoge
Common scab susceptibility of 24 most popular potato cultivars in USA, utilizing a greenhouse assay with three different pathogenic Streptomyces strains (species) Increasing disease score 0 100 200 300 400 500 600 0 100 200 300 400 500 600 0 100 200 300 400 500 600 Norland No data R Norkotah (ND) R Norkotah (ID) Shepody R Norkotah (ND) Ranger Russet No data R Norkotah (ID) R Norkotah 296 R Norkotah ID Norkotah 3 Red La Soda Shepody Yukon Gold Norkotah 8 Shepody Premier Russet Alturas Norkotah 8 Pike Premier Russet Dk Red Norland Norland Yukon Gold Norkotah 3 Russet Burbank Red La Soda Atlantic R Norkotah 296 Russet Burbank Ranger Russet Gold Rush Dk Red Norland Red La Soda Alturas R Norkotah 296 Megachip Snowden Superior Atlantic Superior Yukon Gold Snowden Russet Burbank Megachip Silverton russet Megachip Rio Grande Yukon Gold ME Dakota Pearl Atlantic Canela russet Dakota Pearl Premier Russet Yukon Gold (ID) Norkotah 3 Norland Dakota Pearl Snowden Silverton russet Superior Canela russet Dk Red Norland Pike R Norkotah ND Yukon Gold (WI) S. scabies Blazer Russet S. stelliscabiei Gold Rush S. species IdX Pike Rio Grande Alturas ME01-11h NY02-1c ID01-12c Gold Rush Yukon Gold 5.1e8 CFU/pot Norkotah 8 1.2e9 CFU/pot Blazer Russet 1e9 CFU/pot Ranger Russet Silverton russet Rio Grande Canela russet Blazer Russet Cultivars are listed along the left side of graphs, ranked by disease severity, with most susceptible at the top and most resistant at the bottom. Disease score is a combination of type of lesion (surface, pits or raised lesions) and amount of surface area affected. -

Potato Glossary
A Potato Glossary A Potato Glossary by Richard E. Tucker Last revised 15 Sep 2016 Copyright © 2016 by Richard E. Tucker Introduction This glossary has been prepared as a companion to A Potato Chronology. In that work, a self-imposed requirement to limit each entry to a single line forced the use of technical phrases, scientific words, jargon and terminology that may be unfamiliar to many, even to those in the potato business. It is hoped that this glossary will aid those using that chronology, and it is hoped that it may become a useful reference for anyone interested in learning more about potatoes, farming and gardening. There was a time, a century or more ago, when nearly everyone was familiar with farming life, the raising of potatoes in particular and the lingo of farming in general. They were farmers themselves, they had relatives who farmed, they knew someone who was a farmer, or they worked on a nearby farm during their youth. Then, nearly everyone grew potatoes in their gardens and sold the extra. But that was a long ago time. Now the general population is now separated from the farm by several generations. Only about 2 % of the US population lives on a farm and only a tiny few more even know anyone who lives on a farm. Words and phrases used by farmers in general and potato growers in particular are now unfamiliar to most Americans. Additionally, farming has become an increasingly complex and technical endeavor. Research on the cutting edge of science is leading to new production techniques, new handling practices, new varieties, new understanding of plant physiology, soil and pest ecology, and other advances too numerous to mention. -
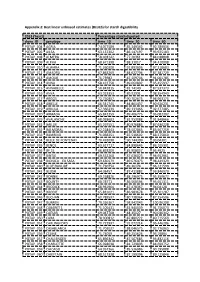
Percentage Starch Digested Clone ID
Appendix 2: Best linear unbiased estimates (BLUES) for starch digestibility 2013 (Year1) Percentage starch digested clone_ID genotype time_10 time_20 time_60 POTAP_004 AGRIA 74.077589 85.349342 92.789936 POTAP_005 AILSA 63.137302 86.147197 93.925183 POTAP_008 ALMERA 58.028142 77.20361 84.038808 POTAP_009 ALPHA 68.671398 88.31017 94.410008 POTAP_010 ALWARA 71.260208 92.893806 97.218683 POTAP_011 AMADRA 67.863269 84.622794 87.381729 POTAP_013 AMOUR 71.73982 87.109706 91.001785 POTAP_014 ANNA 68.422758 89.660892 95.455937 POTAP_015 ANNABELLE 58.843815 82.74149 87.537373 POTAP_016 ANYA 63.923353 89.92991 98.354949 POTAP_017 ARGOS 72.750357 90.702675 98.963175 POTAP_018 ARIELLE 66.341745 84.861437 90.926231 POTAP_019 ARKULA 67.366239 89.333466 92.377485 POTAP_020 ARMADA 65.842359 89.406775 95.082302 POTAP_029 AVALANCHE 68.038683 81.953088 91.848666 POTAP_031 BAILLIE 65.297551 80.976885 87.636228 POTAP_032 BALMORAL 62.228443 78.502863 83.567259 POTAP_033 BAMBINO 71.038251 87.518602 88.375869 POTAP_034 BELLE DE FONTENAY 70.492098 90.744399 93.04301 POTAP_035 BENOL 63.617717 84.898645 86.105301 POTAP_036 BF 15 66.893209 85.908057 93.4925 POTAP_037 BINTJE 69.532268 87.636023 94.952961 POTAP_038 BIONICA EX SASA 69.630179 89.676473 98.449329 POTAP_040 BLUE DANUBE 71.730754 89.36174 96.121599 POTAP_041 BLUSH 64.03457 87.411882 93.846979 POTAP_042 BONNIE 69.258826 88.296975 95.359672 POTAP_043 BOUNTY 68.78771 86.568594 95.651311 POTAP_044 BRODICK 68.961964 87.478367 94.04128 POTAP_045 BRODIE 65.831021 86.983678 95.301287 POTAP_046 BUCHAN 60.789597 -

A Foodservice Guide to Fresh Potato Types POTATOES
THE PERFECT POTATO A Foodservice Guide to Fresh Potato Types POTATOES. THE PERFECT CANVAS FOR MENU INNOVATION. Potatoes aren’t just popular: they’re the #1 side dish in foodservice. You know that baked, mashed, roasted or fried, they have the remarkable ability to sell whatever you serve with them, enhancing presentations and adding value and appetite appeal. Now that you can tap into the intriguing shapes, colors and flavors of today’s exciting new potato types, innovation is easier than ever. No wonder so many chefs, from casual to fine dining, are reinventing potatoes in fresh new ways. So go ahead, grab a handful of potatoes and start thinking big. 1 A SPUD FOR ALL SEASONS AND REASONS. There are more than 4,000 potato varieties worldwide—though only a small fraction is commercialized. In the U.S., about 100 varieties are sold throughout the year to consistently meet the needs of the market. All of these varieties fit into one of five potato type categories: russet, red, white, yellow and specialty (including blue/purple, fingerling and petite). GET CREATIVE! We’ve created these at-a-glance potato type charts to help you find the right potato for any culinary purpose. Use them as a source of inspiration. Even when a potato type is recommended for a given application, another type may also work well to create a similar effect—or even a completely different, yet equally appealing result. Experiment with the types you have access to in new ways you haven’t tried. That’s the key to true menu innovation. -

'Umatilla Russet· and 'Russet Legend· Potato Yield and Quality Response
HORTSCIENCE 38(6):1117–1121. 2003. soil water potential could provide the level of precision necessary to successfully deficit irrigate potato. ‘Umatilla Russet· and ‘Russet Legend· The objective of this research was to com- pare two new cultivars, ‘Umatilla Russet·and Potato Yield and Quality Response to ‘Russet Legend·, with four commercial pro- cessing cultivars to determine the tuber yield Irrigation and quality response to deficit irrigation. The commercial cultivars tested were ‘Russet C.C. Shock1, E.B.G. Feibert2, and L.D. Saunders3 Burbank·, ‘Shepody·, ‘Frontier Russet·, and Malheur Experiment Station, Oregon State Univesity, 595 Onion Avenue, ‘Ranger Russet·. Ontario, OR 97914 Materials and Methods S.R. James4 The trials were conducted in three succes- Central Oregon Agricultural Research Center, Madras, OR 97741 sive years on an Owyhee silt loam (coarse- silty, mixed, mesic, Xerollic Camborthids) at Additional index words. Solanum tuberosum, soil water potential, evapotranspiration, the Oregon State Univ. Malheur Experiment ‘Russet Burbank·, ‘Shepody·, ‘Frontier Russet·, ‘Ranger Russet· Station, in Ontario, Oregon. Potatoes followed alfalfa in 1992, and spring wheat in 1993 and Abstract. ‘Umatilla Russet· and ‘Russet Legend·, two newly released potato (Solanum tuberosum L.) cultivars were compared with four established cultivars (‘Russet Bur- 1994. Fields were bedded into 0.9 m wide hills in the fall of each year. In late April tuber seed bank·, ‘Shepody·, ‘Frontier Russet·, and ‘Ranger Russet·). Potatoes were grown under four, season-long, sprinkler irrigation treatments in three successive years (1992–94) on pieces (60g) were planted at 0.23 m spacing. Residual soil nitrate-N plus ammonium-N in silt loam soil in eastern Oregon. -

Seed Potatoes You Would Like to Request from the Plant Materials Center (PMC) to Plant on Your Farm in 2019
5310 S. Bodenburg Spur Palmer, Alaska 99645-7646 Main: 907.745.4469 Fax: 907.746-1568 November 30, 2017 Dear Grower, Please consider the varieties and quantities of generation zero (G0) seed potatoes you would like to request from the Plant Materials Center (PMC) to plant on your farm in 2019. In order to establish our greenhouse production plans for 2018 at the PMC, we will accept order requests through January 31, 2018. We encourage you to renew your seed stocks as often as possible with disease free seed from the PMC to maintain high quality seed in Alaska potato production. In this regard, we are here to serve you and provide the industry with a healthy start. Please review the attached list and visit http://plants.alaska.gov/PotatoSeedProduction.html for ordering information. If you do not see a variety on the list that interests you, please contact us to see if we can produce the variety or a similar one. Based on production logistics, we are setting a minimum order limit of two pounds per variety. The price per pound is $15.00. Please do not hesitate to contact me with any questions. Sincerely, Christine Macknicki Potato Program Technician (907) 745-8021 [email protected] Available Public Varieties 8-3(Magic Myrna) Butte Favorite Red Maris Piper Rosa 22-1(Delta Red) Cal White French Fingerling Mark Varshaw Rose Finn Apple 29-6 (Fiesta) CalWhite Russet Frontier Russet Mirton Pearl Rose Gold AC Red Island Candy Cane German Butterball Mrs. Moehlers Yellow Royal Kidney AK114 Candystripe Goldrush Myatts Ashleaf Russet Burbank -

Evaluation of 18 Specialty Potatoes in Southwest Michigan Dr
Midwest Vegetable Trial Report for 2014 Evaluation of 18 Specialty Potatoes in Southwest Michigan Dr. Ron Goldy and Virginia Wendzel Southwest Michigan Research and Extension Center Benton Harbor, Michigan Objective The purpose of this trial is to evaluate the performance of 18 specialty potato selections for their adaptability to southwest Michigan growing conditions. This is the third year for evaluating specialty potatoes in an attempt to encourage farmer’s market vendors to make them part of their offerings. Summary Nineteen potato cultivars were evaluated for their production potential in southwest Michigan. A range in yield and tuber quality was found in the entries. Entries included russets, fingerlings, and other tuber types; therefore, the trial as a whole was not subjected to statistical analysis. The three russet tuber types (Rio Grande Russet, Canela Russet, and Russet Burbank) were subjected to analysis. Rio Grande Russet had a statistically higher total yield compared to Russet Burbank. The three entries were similar in other traits evaluated. Methods Fertilizer Prior to planting, polymer-coated urea (44-0-0), 0-0-60, 95% sulfur, and Granubor were broadcast and incorporated at 70, 200, 28, and 15 pounds/acre, respectively. After planting, nutrients were applied through a drip system using Nitro Plus (18N-5Ca-1.5Mg and a proprietary growth regulator) and 28% nitrogen (28-0-0). Nitro Plus was applied at 15 gallons per acre on June 16, 23, 30, and July 7. The 28% was applied July 14, 21, 28, August 3, 11, and 18 for a total nitrogen rate of 164 pounds per acre.