Status and Strategy for Glossy Buckthorn (Frangula Alnus Mill.) Management
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
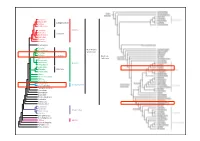
Systematikss11 8.Pdf
Asterales Dipsacales Campanulanae Apiales Aquifoliales Lamiales Asteridae Solanales Lamianae Gentianales Garryales Ericales Cornales Saxifragales Fagales Kern-Eudico- Cucurbitales tyledoneae Rosales Fabales Fabanae Eudicoty- Zygophyllales ledoneae Celastrales Oxalidales Rosidae Malpighiales Sapindales Malvales Malvanae Brassicales Vitales Crossosomatales Myrtales Geraniales Polygonales Caryophyllidae Caryophyllales Berberidopsidales Santalales Gunnerales Buxaceae Trochodendrales Proteales Sabiaceae Ranunculales Canellales Piperales Magnoliidae Magnoliales Laurales Chloanthaceae Ceratophyllaceae Liliidae Liliidae Austrobaileyales Nymphaeales Amborellales Klasse: Magnoliopsida (Angiospermae) Dicotyledoneae, Zweikeimblättrige Ordnung: Rosales Familie: Rhamnaceae (Kreuzdorngewächse) Merkmale: – Holzpflanzen mit wechselständigen oder gegenständigen, ungeteilten Blättern mit krautigen oder dornigen Nebenblättern – radiäre, kleine, gelblich-grüne, meist zwittrige Blüten – meist 5-zählige, selten 4-zählige Blüten – epipetal (= vor den Kronblättern stehende) Staubblätter – intrastaminaler Diskus – Perianth perigyn = mittelständiger Fruchtknoten, selten unterständig – Steinfrüchte, Nussfrüchte oder Spaltfrüchte Klasse: Magnoliopsida (Angiospermae) Dicotyledoneae, Zweikeimblättrige Ordnung: Rosales Familie: Rhamnaceae (Kreuzdorngewächse) Rhamnus frangula = Frangula alnus (Faulbaum) Kelchblatt Kronblatt Merkmale: – 5-zählige Blüte Staubblatt – Rinde mit grauweißen Korkwarzen (= Lentizellen) – Blätter elliptisch-eiförmig, ganzrandig – beerenähnliche -

The Role of the Brown Bear Ursus Arctos As Seed Disperser: a Case Study with the Bilberry Vaccinium Myrtillus
The role of the brown bear Ursus arctos as seed disperser: a case study with the bilberry Vaccinium myrtillus Rola niedźwiedzia brunatnego Ursus arctos w rozprzestrzenianiu nasion: studium przypadku na przykładzie borówki czarnej Vaccinium myrtillus PhD thesis Alberto García-Rodríguez Kraków, 2021 To the memory of José Ignacio and Javier Rodríguez Val Female brown bear with two cubs of the year feeding on bilberry fruits in Tatra National Park (July 2020) “They thought they were burying you, they did not know they were burying a seed” Ernesto Cardenal, Nicaraguan priest, poet and politician PhD CANDIDATE mgr. ALBERTO GARCÍA-RODRÍGUEZ Institute of Nature Conservation of the Polish Academy of Sciences Al. Adama Mickiewicza 33, 31-120, Krakow, Poland SUPERVISOR dr. hab. NURIA SELVA FERNÁNDEZ Institute of Nature Conservation of the Polish Academy of Sciences Al. Adama Mickiewicza 33, 31-120, Krakow, Poland CO-SUPERVISOR dr. JÖRG ALBRECHT Senckenberg Biodiversity and Climate Research Centre (SBiK-F) Senckenberganlage 25, 60325, Frankfurt am Main, Germany. The PhD thesis was prepared during doctoral studies in the Doctoral Study of Natural Sciences of the Polish Academy of Sciences in Kraków. CONTENTS SUMMARY…………..……………..…………………………...………………………………………………...5 STRESZCZENIE……...………….……………………………………………………………………………….8 INTRODUCTION……………………...………………………………………………….……………………...11 PAPER I The role of the brown bear Ursus arctos as a legitimate megafaunal seed disperser………………..…30 PAPER II The bear-berry connection: ecological and management implications of -

Invasion of Transition Hardwood Forests by Exotic Rhamnus Frangula: Chronology and Site Requirements
University of New Hampshire University of New Hampshire Scholars' Repository Master's Theses and Capstones Student Scholarship Spring 2007 Invasion of transition hardwood forests by exotic Rhamnus frangula: Chronology and site requirements Hanna S. Wingard University of New Hampshire, Durham Follow this and additional works at: https://scholars.unh.edu/thesis Recommended Citation Wingard, Hanna S., "Invasion of transition hardwood forests by exotic Rhamnus frangula: Chronology and site requirements" (2007). Master's Theses and Capstones. 286. https://scholars.unh.edu/thesis/286 This Thesis is brought to you for free and open access by the Student Scholarship at University of New Hampshire Scholars' Repository. It has been accepted for inclusion in Master's Theses and Capstones by an authorized administrator of University of New Hampshire Scholars' Repository. For more information, please contact [email protected]. INVASION OF TRANSITION HARDWOOD FORESTS BY EXOTIC RHAMNUS FRANGULA: CHRONOLOGY AND SITE REQUIREMENTS BY HANNA S. WINGARD Baccalaureate Degree, University of Michigan, 2000 THESIS Submitted to the University of New Hampshire in Partial Fulfillment of the Requirements for the Degree of M aster of Science in Natural Resources May, 2007 Reproduced with permission of the copyright owner. Further reproduction prohibited without permission. UMI Number: 1443642 INFORMATION TO USERS The quality of this reproduction is dependent upon the quality of the copy submitted. Broken or indistinct print, colored or poor quality illustrations and photographs, print bleed-through, substandard margins, and improper alignment can adversely affect reproduction. In the unlikely event that the author did not send a complete manuscript and there are missing pages, these will be noted. -

Glossy Buckthorn Rhamnusoriental Frangula Bittersweet / Frangula Alnus Control Guidelines Fact Sheet
Glossy buckthorn Oriental bittersweet Rhamnus frangula / Frangula alnus Control Guidelines Fact Sheet NH Department of Agriculture, Markets & Food, Division of Plant Industry, 29 Hazen Dr, Concord, NH 03301 (603) 271-3488 Common Name: Glossy buckthorn Latin Name: Rhamnus frangula / Frangula alnus New Hampshire Invasive Species Status: Prohibited (Agr 3800) Native to: Japan leaves (spring) Glossy buckthorn invasion Sapling (summer) Flowers (spring) Roadside invasion of saplings Fleshy fruits (fall) Gray bark w/ lenticels (Summer) Emodin in berries - effects to birds Fall foliage (Autumn) Description: Deciduous shrub or small tree measuring 20' by 15'. Bark: Grayish to brown with raised lenticels. Stems: Cinnamon colored with light gray lenticels. Leaves: Alternate, simple and broadly ovate. Flowers: Inconspicuous, 4- petaled, greenish-yellow, mid-May. Fruit: Fleshy, 1/4” diameter turning black in the fall. Zone: 3-7. Habitat: Adapts to most conditions including pH, heavy shade to full sun. Spread: Seeds are bird dispersed. Comments: Highly Aggressive, fast growing, outcompetes native species. Controls: Remove seedlings and saplings by hand. Larger trees can be cut or plants can be treated with an herbicide. General Considerations Glossy buckthorn can either grow as a multi-stemmed shrub or single-stemmed tree up to 23’ (7 m) tall. Leaves are deciduous, simple, and generally arranged alternately. Leaves are dark-green and glossy above while dull-green below. The leaf margins are smooth/entire and tend to be slightly wavy. Flowers are small, about ¼” and somewhat inconspicuous forming in May to June. They develop and in small clusters of 2-8. Fruits form in mid to late summer and contain 2-3 seeds per berry. -

Biology of a Rust Fungus Infecting Rhamnus Frangula and Phalaris Arundinacea
Biology of a rust fungus infecting Rhamnus frangula and Phalaris arundinacea Yue Jin USDA-ARS Cereal Disease Laboratory University of Minnesota St. Paul, MN Reed canarygrass (Phalaris arundinacea) Nature Center, Roseville, MN Reed canarygrass (Phalaris arundinacea) and glossy buckthorn (Rhamnus frangula) From “Flora of Wisconsin” Ranked Order of Terrestrial Invasive Plants That Threaten MN -Minnesota Terrestrial Invasive Plants and Pests Center Puccinia coronata var. hordei Jin & Steff. ✧ Unique spore morphology: ✧ Cycles between Rhamnus cathartica and grasses in Triticeae: o Hordeum spp. o Secale spp. o Triticum spp. o Elymus spp. ✧ Other accessory hosts: o Bromus tectorum o Poa spp. o Phalaris arundinacea Rust infection on Rhamnus frangula Central Park Nature Center, Roseville, MN June 2017 Heavy infections on Rhamnus frangula, but not on Rh. cathartica Uredinia formed on Phalaris arundinacea soon after mature aecia released aeiospores from infected Rhamnus frangula Life cycle of Puccinia coronata from Nazaredno et al. 2018, Molecular Plant Path. 19:1047 Puccinia coronata: a species complex Forms Telial host Aecial host (var., f. sp.) (primanry host) (alternate host) avenae Oat, grasses in Avenaceae Rhamnus cathartica lolii Lolium spp. Rh. cathartica festucae Fescuta spp. Rh. cathartica hoci Hocus spp. Rh. cathartica agronstis Agrostis alba Rh. cathartica hordei barley, rye, grasses in triticeae Rh. cathartica bromi Bromus inermis Rh. cathartica calamagrostis Calamagrostis canadensis Rh. alnifolia ? Phalaris arundinacea Rh. frangula Pathogenicity test on cereal crop species using aeciospores from Rhamnus frangula Cereal species Genotypes Response Oats 55 Immune Barley 52 Immune Wheat 40 Immune Rye 6 Immune * Conclusion: not a pathogen of cereal crops Pathogenicity test on grasses Grass species Genotypes Response Phalaris arundinacea 12 Susceptible Ph. -

FLORA from FĂRĂGĂU AREA (MUREŞ COUNTY) AS POTENTIAL SOURCE of MEDICINAL PLANTS Silvia OROIAN1*, Mihaela SĂMĂRGHIŢAN2
ISSN: 2601 – 6141, ISSN-L: 2601 – 6141 Acta Biologica Marisiensis 2018, 1(1): 60-70 ORIGINAL PAPER FLORA FROM FĂRĂGĂU AREA (MUREŞ COUNTY) AS POTENTIAL SOURCE OF MEDICINAL PLANTS Silvia OROIAN1*, Mihaela SĂMĂRGHIŢAN2 1Department of Pharmaceutical Botany, University of Medicine and Pharmacy of Tîrgu Mureş, Romania 2Mureş County Museum, Department of Natural Sciences, Tîrgu Mureş, Romania *Correspondence: Silvia OROIAN [email protected] Received: 2 July 2018; Accepted: 9 July 2018; Published: 15 July 2018 Abstract The aim of this study was to identify a potential source of medicinal plant from Transylvanian Plain. Also, the paper provides information about the hayfields floral richness, a great scientific value for Romania and Europe. The study of the flora was carried out in several stages: 2005-2008, 2013, 2017-2018. In the studied area, 397 taxa were identified, distributed in 82 families with therapeutic potential, represented by 164 medical taxa, 37 of them being in the European Pharmacopoeia 8.5. The study reveals that most plants contain: volatile oils (13.41%), tannins (12.19%), flavonoids (9.75%), mucilages (8.53%) etc. This plants can be used in the treatment of various human disorders: disorders of the digestive system, respiratory system, skin disorders, muscular and skeletal systems, genitourinary system, in gynaecological disorders, cardiovascular, and central nervous sistem disorders. In the study plants protected by law at European and national level were identified: Echium maculatum, Cephalaria radiata, Crambe tataria, Narcissus poeticus ssp. radiiflorus, Salvia nutans, Iris aphylla, Orchis morio, Orchis tridentata, Adonis vernalis, Dictamnus albus, Hammarbya paludosa etc. Keywords: Fărăgău, medicinal plants, human disease, Mureş County 1. -

Seed Dormancy and Consequences for Direct Tree Seeding H
Seed dormancy and consequences for direct tree seeding H. Frochot, Philippe Balandier, A. Sourisseau To cite this version: H. Frochot, Philippe Balandier, A. Sourisseau. Seed dormancy and consequences for direct tree seed- ing. Final COST E47 conference Forest vegetation management towards environmental sustainability, May 2009, Vejle, Denmark. p. 43 - p. 45. hal-00468732 HAL Id: hal-00468732 https://hal.archives-ouvertes.fr/hal-00468732 Submitted on 31 Mar 2010 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Forest Vegetation Management – towards environmental sustainability, N.S. Bentsen (Ed.), Proccedings from the final COST E47 Conference, Vejle, Denmark, 2009/05/5-7, Forest and Landscape Working Papers n° 35-2009, 43-45. Seed dormancy and consequences for direct tree seeding Henri Frochot1), Philippe Balandier2,3), Agnès Sourisseau4) 1) INRA, UMR1092 LERFOB, F-54280 Champenoux, France, [email protected]; [email protected] 2) Cemagref, UR EFNO, Nogent sur Vernisson, France 3) INRA, UMR547 PIAF, Clermont-Ferrand, France 4) Independent Landscaper, Paris, France Key words Afforestation, direct seeding, dormancy, seed, tree Introduction Whereas direct tree seeding was probably used extensively in France in the past, it is currently only employed for the reforestation of Pinus pinaster and some species with big seeds such as oak. -

Frangula Paruensis, a New Name for Rhamnus Longipes Steyermark (Rhamnaceae)
FRANGULA PARUENSIS, A NEW NAME FOR RHAMNUS LONGIPES STEYERMARK (RHAMNACEAE) GERARDO A. AYMARD C.1, 2 Abstract. The new name Frangula paruensis (Rhamnaceae) is proposed to replace the illegitimate homonym Rhamnus longipes Steyermark (1988). Chorological, taxonomic, biogeographical, and habitat notes about this taxon also are provided. Resumen. Se propone Frangula paruensis (Rhamnaceae) como un nuevo nombre para reemplazar el homónimo ilegítimo Rhamnus longipes Steyermark (1988). Se incluye información corológica, taxonómica, biogeográfica, y de hábitats acerca de la especie. Keywords: Frangula, Rhamnus, Rhamnaceae, Parú Massif, Tepuis flora, Venezuela Rhamnus L. and Frangula Miller (Rhamnaceae) have Frangula paruensis is a shrub, ca. 2 m tall, with leaves ca. 150 and ca. 50 species, respectively (Pool, 2013, 2015). ovate, or oblong-ovate, margin subrevolute, repand- These taxa are widely distributed around the world but are crenulate, a slightly elevated tertiary venation on the lower absent in Madagascar, Australia, and Polynesia (Medan and surface, and mature fruiting peduncle and pedicels 1–1.5 Schirarend, 2004). According to Grubov (1949), Kartesz cm long, and fruiting calyx lobes triangular-lanceolate and Gandhi (1994), Bolmgren and Oxelman (2004), and (two main features to separate Frangula from Rhamnus). Pool (2013) the recognition of Frangula is well supported. This species is endemic to the open, rocky savannas On the basis of historical and recent molecular work the on tepui slopes and summits at ca. 2000 m (Steyermark genus is characterized by several remarkable features. Pool and Berry, 2004). This Venezuelan taxon was described (2013: 448, table 1) summarized 11 features to separate the as Rhamnus longipes by Steyermark (1988), without two genera. -

Herb Other Names Re Co Rde D Me Dicinal Us E Re
RECORDED USE RECORDED USE MEDICINAL US AROMATHERA IN COSMETICS IN RECORDED RECORDED FOOD USE FOOD IN Y E P HERB OTHER NAMES COMMENTS Parts Used Medicinally Abelmoschus moschatus Hibiscus abelmoschus, Ambrette, Musk mallow, Muskseed No No Yes Yes Abies alba European silver fir, silver fir, Abies pectinata Yes No Yes Yes Leaves & resin Abies balsamea Balm of Gilead, balsam fir Yes No Yes Yes Leaves, bark resin & oil Abies canadensis Hemlock spruce, Tsuga, Pinus bark Yes No No No Bark Abies sibirica Fir needle, Siberian fir Yes No Yes Yes Young shoots This species not used in aromatherapy but Abies Sibirica, Abies alba Miller, Siberian Silver Fir Abies spectabilis Abies webbiana, Himalayan silver fir Yes No No No Essential Oil are. Leaves Aqueous bark extract which is often concentrated and dried to produce a flavouring. Distilled with Extract, bark, wood, Acacia catechu Black wattle, Black catechu Yes Yes No No vodka to make Blavod (black vodka). flowering tops and gum Acacia farnesiana Cassie, Prickly Moses Yes Yes Yes Yes Ripe seeds pressed for cooking oil Bark, flowers Source of Gum Arabic (E414) and Guar Gum (E412), controlled miscellaneous food additive. Used Acacia senegal Guar gum, Gum arabic No Yes No Yes in foods as suspending and emulsifying agent. Acanthopanax senticosus Kan jang Yes No No No Kan Jang is a combination of Andrographis Paniculata and Acanthopanax Senticosus. Flavouring source including essential oil. Contains natural toxin thujone/thuyone whose levels in flavourings are limited by EU (Council Directive 88/388/EEC) and GB (SI 1992 No.1971) legislation. There are several chemotypes of Yarrow Essential Oil, which is steam distilled from the dried herb. -

Rhamnaceae) Jürgen Kellermanna,B
Swainsona 33: 43–50 (2020) © 2020 Board of the Botanic Gardens & State Herbarium (Adelaide, South Australia) Nomenclatural notes and typifications in Australian species of Paliureae (Rhamnaceae) Jürgen Kellermanna,b a State Herbarium of South Australia, GPO Box 1047, Adelaide, South Australia 5001 Email: [email protected] b The University of Adelaide, School of Biological Sciences, Adelaide, South Australia 5005 Abstract: The nomenclature of the four species of Ziziphus Mill. and the one species of Hovenia Thunb. occurring in Australia is reviewed, including the role of the Hermann Herbarium for the typification of Z. oenopolia (L.) Mill. and Z. mauritiana Lam. Lectotypes are chosen for Z. quadrilocularis F.Muell. and Z. timoriensis DC. A key to species is provided, as well as illustrations for Z. oenopolia, Z. quadrilocularis and H. dulcis Thunb. Keywords: Nomenclature, typification, Hovenia, Ziziphus, Rhamnaceae, Paliureae, Paul Hermann, Carolus Linnaeus, Henry Trimen, Australia Introduction last worldwide overview of the genus was published by Suessenguth (1953). Since then, only regional Rhamnaceae tribe Paliureae Reissek ex Endl. was treatments and revisions have been published, most reinstated by Richardson et al. (2000b), after the first notably by Johnston (1963, 1964, 1972), Bhandari & molecular analysis of the family (Richardson et al. Bhansali (2000), Chen & Schirarend (2007) and Cahen 2000a). It consists of three genera, Hovenia Thunb., et al. (in press). For Australia, the genus as a whole was Paliurus Tourn. ex Mill. and Ziziphus Mill., which last reviewed by Bentham (1863), with subsequent until then were assigned to the tribes Rhamneae regional treatments by Wheeler (1992) and Rye (1997) Horan. -

Floristic Discoveries in Delaware, Maryland, and Virginia
Knapp, W.M., R.F.C. Naczi, W.D. Longbottom, C.A. Davis, W.A. McAvoy, C.T. Frye, J.W. Harrison, and P. Stango, III. 2011. Floristic discoveries in Delaware, Maryland, and Virginia. Phytoneuron 2011-64: 1–26. Published 15 December 2011. ISSN 2153 733X FLORISTIC DISCOVERIES IN DELAWARE, MARYLAND, AND VIRGINIA WESLEY M. KNAPP 1 Maryland Department of Natural Resources Wildlife and Heritage Service Wye Mills, Maryland 21679 [email protected] ROBERT F. C. NACZI The New York Botanical Garden Bronx, New York 10458-5126 WAYNE D. LONGBOTTOM P.O. Box 634 Preston, Maryland 21655 CHARLES A. DAVIS 1510 Bellona Ave. Lutherville, Maryland 21093 WILLIAM A. MCAVOY Delaware Natural Heritage and Endangered Species Program 4876 Hay Point, Landing Rd. Smyrna, Delaware 19977 CHRISTOPHER T. FRYE Maryland Department of Natural Resources Wildlife and Heritage Service Wye Mills, Maryland 21679 JASON W. HARRISON Maryland Department of Natural Resources Wildlife and Heritage Service Wye Mills, Maryland 21679 PETER STANGO III Maryland Department of Natural Resources, Wildlife and Heritage Service, Annapolis, Maryland 21401 1 Author for correspondence ABSTRACT Over the past decade studies in the field and herbaria have yielded significant advancements in the knowledge of the floras of Delaware, Maryland, and the Eastern Shore of Virginia. We here discuss fifty-two species newly discovered or rediscovered or whose range or nativity is clarified. Eighteen are additions to the flora of Delaware ( Carex lucorum var. lucorum, Carex oklahomensis, Cyperus difformis, Cyperus flavicomus, Elymus macgregorii, Glossostigma cleistanthum, Houstonia pusilla, Juncus validus var. validus, Lotus tenuis, Melothria pendula var. pendula, Parapholis incurva, Phyllanthus caroliniensis subsp. -

Conservation Assessment for Arctic Raspberry (Rubus Acaulis) Michx
Conservation Assessment for Arctic Raspberry (Rubus acaulis) Michx. USDA Forest Service, Eastern Region May 2002 This Conservation Assessment/Approach was prepared to compile the published and unpublished information on the subject taxon or community; or this document was prepared by another organization and provides information to serve as a Conservation Assessment for the Eastern Region of the Forest Service. It does not represent a management decision by the U.S. Forest Service. Though the best scientific information available was used and subject experts were consulted in preparation of this document, it is expected that new information will arise. In the spirit of continuous learning and adaptive management, if you have information that will assist in conserving the subject taxon, please contact the Eastern Region of the Forest Service Threatened and Endangered Species Program at 310 Wisconsin Avenue, Suite 580 Milwaukee, Wisconsin 53203. Conservation Assessment for Artic Raspberry (Rubus acaulis) Michx. 2 Table Of Contents INTRODUCTION/OBJECTIVES .........................................................................4 EXECUTIVE SUMMARY .....................................................................................4 ACKNOWLEDGEMENTS ....................................................................................5 NOMENCLATURE AND TAXONOMY..............................................................6 SPECIES DESCRIPTION ......................................................................................7 GEOGRAPHIC