Mercury in Europe's Environment
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Sturgeon Chub (Macrhybopsis Gelida): a Technical Conservation Assessment
Sturgeon Chub (Macrhybopsis gelida): A Technical Conservation Assessment Prepared for the USDA Forest Service, Rocky Mountain Region, Species Conservation Project August 31, 2004 Frank J. Rahel and Laura A. Thel Department of Zoology and Physiology University of Wyoming, Laramie, Wyoming 82071 Peer Review Administered by American Fisheries Society Rahel, F.J. and L.A. Thel. (2004, August 31). Sturgeon Chub (Macrhybopsis gelida): a technical conservation assessment. [Online]. USDA Forest Service, Rocky Mountain Region. Available: http://www.fs.fed.us/r2/ projects/scp/assessments/sturgeonchub.pdf [date of access]. ACKNOWLEDGEMENTS We thank biologists from Colorado, Kansas, Nebraska, South Dakota, and Wyoming, and from the national forests and national grasslands within Region 2 who provided information about sturgeon chub within their jurisdictions. We especially thank Gregory Hayward and Richard Vacirca of the USDA Forest Service for their review of this species assessment. Comments also were provided by two anonymous reviewers. David B. McDonald of the University of Wyoming provided the population demographic matrix analysis. AUTHORS’ BIOGRAPHIES Frank J. Rahel is a professor in the Department of Zoology and Physiology at the University of Wyoming where he teaches courses in fi sheries management, ichthyology, and conservation biology. His research interests are centered around fi sh ecology and the infl uence of anthropogenic disturbances on fi sh assemblages. Laura A. Thel is a graduate research assistant in the Department of Zoology and Physiology at the University of Wyoming with research interests involving stream ecology, hydrology, and landscape ecology, especially as these are related to the management of native fi shes. COVER PHOTO CREDIT Sturgeon Chub (Macrhybopsis gelida). -

IGFA Angling Rules
International Angling Rules The following angling rules have been formulated by the International Game Fish Association to promote ethical and sporting angling practices, to establish uniform regulations for the compilation of world game fish records, and to provide basic angling guidelines for use in fishing tournaments and any other group angling activities. The word “angling” is defined as catching or attempting to catch fish with a rod, reel, line, and hook as outlined in the international angling rules. There are some aspects of angling that cannot be controlled through rule making, however. Angling regulations cannot insure an outstanding performance from each fish, and world records cannot indicate the amount of difficulty in catching the fish. Captures in which the fish has not fought or has not had a chance to fight do not reflect credit on the fisherman, and only the angler can properly evaluate the degree of achievement in establishing the record. Only fish caught in accordance with IGFA international angling rules, and within the intent of these rules, will be considered for world records. Following are the rules for freshwater and saltwater fishing and a separate set of rules for fly fishing. RULES FOR FISHING IN FRESH AND SALT WATER (Also see Rules for Fly fishing) E. ROD Equipment Regulations 1. Rods must comply with sporting ethics and customs. A. LINE Considerable latitude is allowed in the choice of a rod, but rods giving 1. Monofilament, multifilament, and lead core multifilament the angler an unfair advantage will be disqualified. This rule is lines may be used. For line classes, see World Record Requirements. -
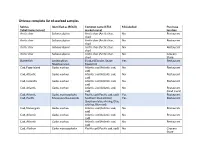
Ottawa: Complete List of Seafood Samples
Ottawa: complete list of seafood samples Sold as Identified as (BOLD) Common name (CFIA Mislabelled Purchase (label/menu/server) market name) location Arctic char Salverus alpirus Arctic char (Arctic char, No Restaurant char) Arctic char Salverus alpirus Arctic char (Arctic char, No Restaurant char) Arctic char Salverus alpirus Arctic char (Arctic char, No Restaurant char) Arctic char Salverus alpirus Arctic char (Arctic char, No Grocery char) Store Butterfish Lepidocybium Escolar (Escolar, Snake Yes Restaurant flavobrunneum Mackerel) Cod, Fogo Island Gadus morhua Atlantic cod (Atlantic cod, No Restaurant cod) Cod, Atlantic Gadus morhua Atlantic cod (Atlantic cod, No Restaurant cod) Cod, Icelandic Gadus morhua Atlantic cod (Atlantic cod, No Restaurant cod) Cod, Atlantic Gadus morhua Atlantic cod (Atlantic cod, No Restaurant cod) (food truck) Cod, Atlantic Gadus macrocephalus Pacific cod (Pacific cod, cod) Yes Restaurant Cod, Pacific Micromesistius australis Southern blue whiting Yes Restaurant (Southern blue whiting, Blue whiting, Blue cod) Cod, Norwegian Gadus morhua Atlantic cod (Atlantic cod, No Restaurant cod) Cod, Atlantic Gadus morhua Atlantic cod (Atlantic cod, No Restaurant cod) Cod, Atlantic Gadus morhua Atlantic cod (Atlantic cod, No Restaurant cod) Cod, Alaskan Gadus macrocephalus Pacific cod (Pacific cod, cod) No Grocery Store Cod, Pacific Gadus macrocephalus Pacific cod (Pacific cod, cod) No Grocery Store Cod, North Atlantic Gadus macrocephalus Pacific cod (Pacific cod, cod) Yes Restaurant Cod Gadus macrocephalus Pacific cod (Pacific cod, cod) No Grocery Store Cod, Icelandic Gadus morhua Atlantic cod (Atlantic cod, No Grocery cod) Store Cod, Icelandic Gadus morhua Atlantic cod (Atlantic cod, No Grocery cod) Store Euro Bass Gadus morhua Atlantic cod (Atlantic cod, Yes Restaurant cod) Grouper Epinephelus diacanthus Spinycheek grouper (n/a) Yes – E. -

Myxosporea: Ceratomyxidae) to Encompass Freshwater Species C
Erection of Ceratonova n. gen. (Myxosporea: Ceratomyxidae) to Encompass Freshwater Species C. gasterostea n. sp. from Threespine Stickleback (Gasterosteus aculeatus) and C. shasta n. comb. from Salmonid Fishes Atkinson, S. D., Foott, J. S., & Bartholomew, J. L. (2014). Erection of Ceratonova n. gen.(Myxosporea: Ceratomyxidae) to Encompass Freshwater Species C. gasterostea n. sp. from Threespine Stickleback (Gasterosteus aculeatus) and C. shasta n. comb. from Salmonid Fishes. Journal of Parasitology, 100(5), 640-645. doi:10.1645/13-434.1 10.1645/13-434.1 American Society of Parasitologists Accepted Manuscript http://cdss.library.oregonstate.edu/sa-termsofuse Manuscript Click here to download Manuscript: 13-434R1 AP doc 4-21-14.doc RH: ATKINSON ET AL. – CERATONOVA GASTEROSTEA N. GEN. N. SP. ERECTION OF CERATONOVA N. GEN. (MYXOSPOREA: CERATOMYXIDAE) TO ENCOMPASS FRESHWATER SPECIES C. GASTEROSTEA N. SP. FROM THREESPINE STICKLEBACK (GASTEROSTEUS ACULEATUS) AND C. SHASTA N. COMB. FROM SALMONID FISHES S. D. Atkinson, J. S. Foott*, and J. L. Bartholomew Department of Microbiology, Oregon State University, Nash Hall 220, Corvallis, Oregon 97331. Correspondence should be sent to: [email protected] ABSTRACT: Ceratonova gasterostea n. gen. n. sp. is described from the intestine of freshwater Gasterosteus aculeatus L. from the Klamath River, California. Myxospores are arcuate, 22.4 +/- 2.6 µm thick, 5.2 +/- 0.4 µm long, posterior angle 45 +/- 24°, with 2 sub-spherical polar capsules, diameter 2.3 +/- 0.2 µm, which lie adjacent to the suture. Its ribosomal small subunit sequence was most similar to an intestinal parasite of salmonid fishes, Ceratomyxa shasta (97%, 1,671/1,692 nt), and distinct from all other Ceratomyxa species (<85%), which are typically coelozoic parasites in the gall bladder or urinary system of marine fishes. -

Why Baumax and Praktiker Disappeared – Why Did They Fail?
Why baumax and Praktiker disappeared – why did they fail? Stockholm, 9th Juni 2016 Prof. Dr.rer.pol. Dr.-Ing. Thomas Roeb M.A. HS (University of Applied Sciences) Bonn-Rhein-Sieg 4th Global DIY-Summit Prof. Dr.rer.pol. Dr.-Ing. Thomas Roeb M.A. 1 9th Juni 2016 HS Bonn-Rhein-Sieg - [email protected] Prof. Dr. Dr. Thomas ROEB M.A. clients (selected retailers) contact • AMS Sourcing B.V. (NL) CEO • atb market (UA) Marketing Director • Anton Schlecker VP Purchasing • basic AG CEO • Delhaize Group (B) Senior VP Group Purchasing • Denner AG (CH) CEO • denree GmbH CEO • dm-drogerie markt GmbH & Co. KG Owner/CEO • EDEKA Zentrale AG & Co. KG Director Marketing • Gebr. Bratzler Fruchtgroßhandel CEO • Ihr Platz GmbH & Co. KG VP Sales and Purchasing • Jawoll GmbH Owner/CEO • Kopeika (RUS) Consultants to the Board • Kaufland Stiftung VP Purchasing • Lidl Discount Stiftung CEO • Nordwest Handel AG 3 different CEOs • Penny Discount CEO • Plus Warenhandels GmbH CEO • Praktiker AG CEO • REWE AG CEO • Tchibo AG VP Sales & Marketing • Weltladendachverband e.V. Head of Marketing 2 Prof. Dr. Dr. Thomas ROEB M.A. clients (selected suppliers): • n.v. artic s.a. • AVO-Werke August Beisse GmbH • Axel-Springer Verlags AG • Bongrain Deutschland GmbH • GlaxoSmithKline Consumer Healthcare … • Hewlett Packard Europe • Intersnack Knabber-Gebäck GmbH & Co. KG • Josef Schmitz Grafschafter Krautfabrik GmbH • Laverana GmbH • Moksel-Gruppe • Nestlé Deutschland AG • Fleischwarenfabrik Heinrich Nölke GmbH & Co • H.&E. Reinert Westfälische Privat-Fleischerei … • SC Johnson GmbH • Westfleisch 3 Prof. Dr. Dr. Thomas ROEB M.A. clients (selected others): • Bundesministerium für Verbraucherschutz, Ernährung und Landwirtschaft (Ministry of Consumer Protection, Food and Agriculture) • CMA Centrale Marketing-Gesellschaft der Deutschen Agrarwirtschaft mbH (Central Marketing Organization of the German Agriculture) • Die Verbraucherinitiative e.V. -

Good Progress on Our Journey 2017/18 Summary Annual Review Financial Highlights
ONE Kingfisher good progress on our journey 2017/18 summary annual review financial highlights Sales1 Retail profit1 Underlying pre-tax profit2 £11,655m £849m £797m (0.3)% (3.6)% +1.3% 2017/18 £11,655m 2017/18 £849m 2017/18 £797m 2016/17 £11,225m 2016/17 £847m 2016/17 £787m Adjusted Lease-adjusted return on Full year pre-tax profit2 capital employed (ROCE) dividend2 £683m 10.4% 10.8p (8.1)% (210)bps +4.0% 2017/18 £683m 2017/18 10.4% 2017/18 10.8p 2016/17 £743m 2016/17 12.5% 2016/17 10.4p Statutory Statutory Basic earnings per share (EPS)2 pre-tax profit2 post-tax profit2 £682m £485m 22.1p (10.1)% (20.5)% (18.5)% 2017/18 £682m 2017/18 £485m 2017/18 22.1p 2016/17 £759m 2016/17 £610m 2016/17 27.1p 1. Percentage change reported on a constant currency basis. 2. Percentage change reported on a reported currency basis. See glossary on page 21 contents 2 Kingfisher at a glance 4 Chief executive officer’s statement 7 Our transformation 8 Our ambition and our purpose 9 Our home improvement ecosystem 10 Our framework for action 11 Progress against our strategic milestones in year 2 14 Our strategic milestones for year 3 15 A clear long-term roadmap 16 People: realising our ambition 18 Becoming a truly sustainable company 20 Group executive 21 Glossary Kingfisher at a glance Kingfisher plc is a home improvement company with Our ambition is to become the leading home improvement nearly 1,300 stores in 10 countries across Europe. -
Including Style Guide and Master Layouts
RESEARCH REPORT Ukraine Part of the CBRE affiliate network Kyiv Retail Market, 2017 Retailers Grasp Leftover Vacant Space As Completions Reach New Low Retail Turnover New Completions Rents Up by Average Vacancy +9.7% y-o-y 8,000 sq m 10-25% y-o-y 5% (-6pp y-o-y) Hot Topics ▪ Continued growth of organized retail turnover (+9.7% y-o-y over January – December) on the back of stronger consumer demand ▪ Record low new completions (8,000 sq m in 2017) due to number of delays ▪ Large volume of new announced supply in 2018-2020 (600,000 sq m), 222,500 sq m of which is announced for delivery in 2018 ▪ Substantial decline in average market vacancy (down to 5%, -6 pp y-o-y) due to scant new supply and rising absorption of previously vacant space ▪ Noticeable rental growth (up by 10-25% y-o-y) as a combination of growing demand and virtually no new supply DEMAND Recovery of Ukrainian retail market is a Occupier demand in 2017 underwent a fresh reflection of the country’s economic revival wave of noticeable growth, fueled by active driven by domestic consumption, which, in expansion of multiple retail chains. Along with turn, was galvanized by gradual rise in real natural expansion, food retailers grew their income over 2017. Kyiv retail turnover market share through acquiring existing continued to expand growing by 9.7% y-o-y over competitors. SPAR Ukraine Corporation (part of January - December and was accompanied by a VolWest Group) signed a corporation somewhat lower than last year increase in real agreement with SPAR International with a view wages (+11.3% vs 14.3%) and a steady pension to develop the supermarket chain in different growth of +5.0% y-o-y. -

Oncorhynchus Mykiss) (Salmoniformes: Salmonidae) Diet in Hawaiian Streams1
Pacific Science (1999), vol. 53, no. 3: 242-251 © 1999 by University of Hawai'i Press. All rights reserved Alien Rainbow Trout (Oncorhynchus mykiss) (Salmoniformes: Salmonidae) Diet in Hawaiian Streams1 MICHAEL H. KIDO,2 DONALD E. HEACOCK,3 AND ADAM ASQUITH4 ABSTRACT: Diet of rainbow trout, Oncorhynchus mykiss (Walbaum), intro duced by the State of Hawai'i into tropical headwater streams of the Waimea River in the Koke'e area ofthe Hawaiian island ofKaua'i, was examined in this study through gut content analysis. In Wai'alae Stream, rainbow trout were found to be opportunistic general predators efficient at feeding on invertebrate drift. Foods eaten ranged from juvenile trout, to terrestrial and aquatic arthro pods, to algae and aquatic mosses. Native aquatic species, particularly dragonfly (Anax strennus) and damselfly (Megalagrion heterogamias) naiads, lyrnnaeid snails (Erinna aulacospira), and atyid shrimp (Atyoida bisulcata), were deter mined to be major foods for alien trout. Terrestrial invertebrates (primarily ar thropods), however, provided a substantial (albeit unpredictable) additional food supply. Based on results of the study, it is cautioned that large numbers of rainbow trout indiscriminantly released into lower- to middle-elevation reaches ofHawaiian streams could do substantial damage to populations ofna tive aquatic species through predation, competition, and/or habitat alteration. RAINBOW TROUT, Oncorhynchus mykiss the high-elevation (ca. 3500 ft [1067 m]) Ko (Walbaum), along with several other Salmo ke'e area of Kaua'i Island (Figure I) (Need niformes (Salmonidae), were introduced into ham and Welsh 1953). By 1941, only Koke'e Hawaiian Island streams early in the century streams were being stocked. -

German Market Analysis and IUU Assessment
German Market Analysis and IUU Assessment WWF Germany [80101064/#15007/jw] Phase 1 Report Final July 2015 Submitted by MRAG Ltd is an independent fisheries and aquatic resource consulting company dedicated to the sustainable use of natural resources through sound, integrated management practices and policies. Established in 1986, MRAG has successfully completed projects in more than 60 countries. Our in-house experts have a wide variety of technical expertise and practical experience across all aspects of aquatic resource management, policy and planning, allowing us to take a multi-disciplinary approach to every project. Our capability to service an extensive array of resource management needs is further extended through our network of associations with internationally acclaimed experts in academic institutions and private organisations worldwide. MRAG Ltd 18 Queen Street T: +44 (0) 20 7255 7755 London F: +44 (0) 20 7499 5388 W1J 5PN W: www.mrag.co.uk United Kingdom E: [email protected] Contents Tables ....................................................................................................................... iii Figures ..................................................................................................................... iii Abbreviations .......................................................................................................... iv 1 Introduction ........................................................................................................ 1 2 Review of German Market Study ..................................................................... -

Supply Chain Excellence in the Retail Industry METRO AG – a Case Study
Supply Chain Excellence in the Retail Industry METRO AG – A Case Study by Manuela Schranz-Whitaker B.B.A. in International Business Golden Gate University, CA 2002 Submitted to the Zaragoza Logistics Center, a Research Institute associated with the University of Zaragoza, in Partial Fulfillment of the Requirements for the Degree of Master of Engineering in Logistics and Supply Chain Management in the MIT-Zaragoza International Logistics Program May 2005 © Manuela Schranz-Whitaker. All rights reserved The author hereby grants to the Massachusetts Institute of Technology (MIT) and the Zaragoza Logistics Center permission to reproduce and to distribute publicly paper and electronic copies of this thesis in whole or in part. Signature of Author ______________________________________________ MIT-Zaragoza International Logistics Program May 20, 2005 Certified by ____________________________________________________ Dr. Jarrod Goentzel, Executive Director MIT-Zaragoza International Logistics Program and ____________________________________________________ Dr. Paul Thompson, Professor MIT-Zaragoza International Logistics Program Accepted by ____________________________________________________ Dr. María Jesús Sáenz, Academic Director MIT-Zaragoza International Logistics Program _____________________________________________________________________ 2 _____________________________________________________________________ 3 Supply Chain Excellence in the Retail Industry METRO AG – A Case Study by Manuela Schranz-Whitaker B.B.A. in International Business -

An Overview of ECISMA and FWC Efforts for Nonnative Fish
An Overview of ECISMA and FWC Efforts For Nonnative Fish ECISMA Summit July 17, 2019 Kelly Gestring, Florida Fish and Wildlife Conservation Commission Jeff Kline, Everglades National Park John Galvez, U.S. Fish and Wildlife Service Pam Schofield, U. S. Geological Service 2019 ECISMA Nonnative Fish RoundUp April 26-27, 2019 • 2 weigh-in locations • 25 Anglers • 1,195 fish • 679 pounds • 18 different species • No new species • Biggest Fish – 7.10 lb Bullseye • Most Species – 11 • Most Weight – 143.8 lbs T-shirts For Sale! Early Detection and Rapid Response Fish Slams = Range expansion, discovery of new species November 2018 Fish Slam, SE Florida • 11 agencies, 32 biologists, 22 sites • 23 nonnative fish species A big shout out to Pam Schofield • No new exotics; uncommon – and Mary Brown, USGS!! common carp Early Detection and Rapid Response Spring 2019 Fish Slam March 2019 Fish Slam; Vero Beach area • 10 agencies, 30 biologists, 18 sites • 11 nonnative fish species • Swordtails (new to area), Rio Grande Cichlid (uncommon) Early Detection and Rapid Response Range expansion, discovery of new species Natural Waters Surveys • North Fork of the St. Lucie River and Lox Slough • No new species detected • Primarily Mayans, Sailfin Catfish and Tilapia Risk Screenings of Fresh and Saltwater Fishes Pomacentrids • >4 million imported annually (40%) • High propagule pressure • 11 species examined • Regal Demoiselle – established in GOM Large-bodied Nonnative Catfish • 2 species monitored in Miami Beach • Redtail Catfish, Goonch, 3 • Bioprofiles completed, FISKv2 next Pseudoplatystoma spp. • Singleton catches of all but Goonch • Bioprofile, FISKv2, LLTs • Spawning requirement issues Source: Jack Randall/USGS. -

A Guide to Building and Managing Private Fish Ponds in Montana
A Guide to Building and Managing Private Fish Ponds in Montana July 2006 Fisheries Division This guide was originally prepared by George Holton, retired Chief Fisheries Biologist and Assistant Administrator of the Fisheries Division. It was revised in 1994 by Joe Urbani and Associates, Inc. and again in 2006 by Sally Schrank under contract with Montana Fish, Wildlife & Parks. Numerous department biologists contributed to earlier versions and participated in this latest revision. TABLE OF CONTENTS Page INTRODUCTION.................................................................................................................1 OVERVIEW ...................................................................................................................... 1-2 I. PLANNING A NEW FISH POND ......................................................................................3 Legal Requirements...............................................................................................................3 Water Quantity 4 Water Quality.........................................................................................................................5 Watershed Analysis...............................................................................................................6 Soil Analysis..........................................................................................................................6 Overview................................................................................................................................6