Oncorhynchus Mykiss) (Salmoniformes: Salmonidae) Diet in Hawaiian Streams1
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Sturgeon Chub (Macrhybopsis Gelida): a Technical Conservation Assessment
Sturgeon Chub (Macrhybopsis gelida): A Technical Conservation Assessment Prepared for the USDA Forest Service, Rocky Mountain Region, Species Conservation Project August 31, 2004 Frank J. Rahel and Laura A. Thel Department of Zoology and Physiology University of Wyoming, Laramie, Wyoming 82071 Peer Review Administered by American Fisheries Society Rahel, F.J. and L.A. Thel. (2004, August 31). Sturgeon Chub (Macrhybopsis gelida): a technical conservation assessment. [Online]. USDA Forest Service, Rocky Mountain Region. Available: http://www.fs.fed.us/r2/ projects/scp/assessments/sturgeonchub.pdf [date of access]. ACKNOWLEDGEMENTS We thank biologists from Colorado, Kansas, Nebraska, South Dakota, and Wyoming, and from the national forests and national grasslands within Region 2 who provided information about sturgeon chub within their jurisdictions. We especially thank Gregory Hayward and Richard Vacirca of the USDA Forest Service for their review of this species assessment. Comments also were provided by two anonymous reviewers. David B. McDonald of the University of Wyoming provided the population demographic matrix analysis. AUTHORS’ BIOGRAPHIES Frank J. Rahel is a professor in the Department of Zoology and Physiology at the University of Wyoming where he teaches courses in fi sheries management, ichthyology, and conservation biology. His research interests are centered around fi sh ecology and the infl uence of anthropogenic disturbances on fi sh assemblages. Laura A. Thel is a graduate research assistant in the Department of Zoology and Physiology at the University of Wyoming with research interests involving stream ecology, hydrology, and landscape ecology, especially as these are related to the management of native fi shes. COVER PHOTO CREDIT Sturgeon Chub (Macrhybopsis gelida). -

The Phylogeny of Oncorhynchus (Euteleostei: Salmonidae) Based on Behavioral and Life History Characters
Copeia, 2007(3), pp. 520–533 The Phylogeny of Oncorhynchus (Euteleostei: Salmonidae) Based on Behavioral and Life History Characters MANU ESTEVE AND DEBORAH A. MCLENNAN There is no consensus between morphological and molecular data concerning the relationships within the Pacific basin salmon and trout clade Oncorhynchus. In this paper we add another source of characters to the discussion. Phylogenetic analysis of 39 behavioral and life history traits produced one tree structured (O. clarki (O. mykiss (O. masou (O. kisutch (O. tshawytscha (O. nerka (O. keta, O. gorbuscha))))))). This topology is congruent with the phylogeny based upon Bayesian analysis of all available nuclear and mitochondrial gene sequences, with the exception of two nodes: behavior supports the morphological data in breaking the sister-group relationship between O. mykiss and O. clarki, and between O. kisutch and O. tshawytscha. The behavioral traits agreed with molecular rather than morphological data in placing O. keta as the sister-group of O. gorbuscha. The behavioral traits also resolve the molecular-based ambiguity concerning the placement of O. masou, placing it as sister to the rest of the Pacific basin salmon. Behavioral plus morphological data placed Salmo, not Salvelinus, as more closely related to Oncorhynchus, but that placement was only weakly supported and awaits collection of missing data from enigmatic species such as the lake trout, Salvelinus namaycush. Overall, the phenotypic characters helped resolve ambiguities that may have been created by molecular introgression, while the molecular traits helped resolve ambiguities introduced by phenotypic homoplasy. It seems reasonable therefore, that systematists can best respond to the escalating biodiversity crisis by forming research groups to gather behavioral and ecological information while specimens are being collected for morphological and molecular analysis. -

IGFA Angling Rules
International Angling Rules The following angling rules have been formulated by the International Game Fish Association to promote ethical and sporting angling practices, to establish uniform regulations for the compilation of world game fish records, and to provide basic angling guidelines for use in fishing tournaments and any other group angling activities. The word “angling” is defined as catching or attempting to catch fish with a rod, reel, line, and hook as outlined in the international angling rules. There are some aspects of angling that cannot be controlled through rule making, however. Angling regulations cannot insure an outstanding performance from each fish, and world records cannot indicate the amount of difficulty in catching the fish. Captures in which the fish has not fought or has not had a chance to fight do not reflect credit on the fisherman, and only the angler can properly evaluate the degree of achievement in establishing the record. Only fish caught in accordance with IGFA international angling rules, and within the intent of these rules, will be considered for world records. Following are the rules for freshwater and saltwater fishing and a separate set of rules for fly fishing. RULES FOR FISHING IN FRESH AND SALT WATER (Also see Rules for Fly fishing) E. ROD Equipment Regulations 1. Rods must comply with sporting ethics and customs. A. LINE Considerable latitude is allowed in the choice of a rod, but rods giving 1. Monofilament, multifilament, and lead core multifilament the angler an unfair advantage will be disqualified. This rule is lines may be used. For line classes, see World Record Requirements. -

Rainbow Trout
Aboriginal Aquaculture Association • FinfishFinfish Facts Facts Rainbow Trout Rainbow Trout (Oncorhynchus mykiss - Latin name) The rainbow trout is a species of salmonid native to tributaries of the Pacific Ocean in Asia and North America. The steelhead is a sea-run rainbow trout (anadromous) usually returning to freshwater to spawn after two to three years at sea; rainbow trout and steelhead trout are the same species. The fsh are often called salmon trout. Several other fish in the salmonid family are called trout; some are anadromous like salmon, whereas others are resident in freshwater only. The species has been introduced for food or sport to many countries, and every continent except Antarctica. The first rainbow trout hatchery was established on San Leandro Creek, a tributary of San Francisco Bay, in 1870, with trout production beginning in 1871. Today, they are farmed in many countries throughout the world. Since the 1950s, commercial production has grown exponentially, particularly in Europe and recently in Chile. In Chile and Norway, ocean cage production of steelheads has expanded to supply export markets. Inland production of rainbow trout to supply domestic markets has increased in countries such as Italy, France, Germany, Denmark and Spain. Other significant producing countries include the USA, Iran, Germany and the United Kingdom. Rainbow Trout Farming in BC Rainbow trout fillets Nutrition Facts for Rainbow Trout: per 3.5 oz (100 g) cooked weight Energy 131 calories Protein 18.4 g Total fat 5.8 g Trout Production • 2010 Saturated fat .09 g Cholesterol 56.0 mg Total carbohydrates 0 g Sodium 39.0 mg Omega-3 1.1 g Source: Seafood Business Rainbow Trout Life Cycle: Alevins Rainbow Trout begin their lives at a domestic Brood Stock facility. -

Edna Assay Development
Environmental DNA assays available for species detection via qPCR analysis at the U.S.D.A Forest Service National Genomics Center for Wildlife and Fish Conservation (NGC). Asterisks indicate the assay was designed at the NGC. This list was last updated in June 2021 and is subject to change. Please contact [email protected] with questions. Family Species Common name Ready for use? Mustelidae Martes americana, Martes caurina American and Pacific marten* Y Castoridae Castor canadensis American beaver Y Ranidae Lithobates catesbeianus American bullfrog Y Cinclidae Cinclus mexicanus American dipper* N Anguillidae Anguilla rostrata American eel Y Soricidae Sorex palustris American water shrew* N Salmonidae Oncorhynchus clarkii ssp Any cutthroat trout* N Petromyzontidae Lampetra spp. Any Lampetra* Y Salmonidae Salmonidae Any salmonid* Y Cottidae Cottidae Any sculpin* Y Salmonidae Thymallus arcticus Arctic grayling* Y Cyrenidae Corbicula fluminea Asian clam* N Salmonidae Salmo salar Atlantic Salmon Y Lymnaeidae Radix auricularia Big-eared radix* N Cyprinidae Mylopharyngodon piceus Black carp N Ictaluridae Ameiurus melas Black Bullhead* N Catostomidae Cycleptus elongatus Blue Sucker* N Cichlidae Oreochromis aureus Blue tilapia* N Catostomidae Catostomus discobolus Bluehead sucker* N Catostomidae Catostomus virescens Bluehead sucker* Y Felidae Lynx rufus Bobcat* Y Hylidae Pseudocris maculata Boreal chorus frog N Hydrocharitaceae Egeria densa Brazilian elodea N Salmonidae Salvelinus fontinalis Brook trout* Y Colubridae Boiga irregularis Brown tree snake* -

Sport-Fish-Identification.Pdf
Walleye Walleye have two distinct fins on their back, the first with large spines. Lake Sturgeon They have a yellow-olive back, brassy, silvery sides with yellow spots, a white underside, and white on the lower lobe of the tail. Dusky vertical Lake Sturgeon are a Threatened Species due to population size and bars are often found on the body as well. concerns with viability. Lake Sturgeon have a large brown or grey body covered with tough, leather- like tissue and five rows of bony plates. They have a shark-like, upturned tail and a pointed snout with four barbels. Sauger Lake Whitefish are olive-green to blue on the back, with silvery sides.They Sauger are a Threatened Species due to hybridization, habitat Lakehave a small Whitefish mouth below a rounded snout, and a deeply forked tail. degradation and overharvest. Sauger are golden olive on the back with silver-yellow sides and a white underside. They also have a large spiny dorsal fin, distinct rows of spots on the dorsal fins and three or four dusky vertical bars on the body. Mountain Whitefish have large scales, no spots and small mouths with no Burbot Mountainteeth. Their general Whitefish body colour is a bronze-white or greenish white. Burbot have a slim, brownish black body with smooth skin, a flattened head, and a fin that stretches along the back half of the body. Distinctive barbels hang from the lower jaw and nostrils. Goldeye Northern Pike Goldeye have prominent eyes with bright yellow pupils, a blunt head, and Northern Pike are a long, slender fish with duck-like jaws and a long, flat a deep, compressed body. -
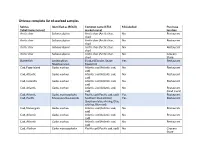
Ottawa: Complete List of Seafood Samples
Ottawa: complete list of seafood samples Sold as Identified as (BOLD) Common name (CFIA Mislabelled Purchase (label/menu/server) market name) location Arctic char Salverus alpirus Arctic char (Arctic char, No Restaurant char) Arctic char Salverus alpirus Arctic char (Arctic char, No Restaurant char) Arctic char Salverus alpirus Arctic char (Arctic char, No Restaurant char) Arctic char Salverus alpirus Arctic char (Arctic char, No Grocery char) Store Butterfish Lepidocybium Escolar (Escolar, Snake Yes Restaurant flavobrunneum Mackerel) Cod, Fogo Island Gadus morhua Atlantic cod (Atlantic cod, No Restaurant cod) Cod, Atlantic Gadus morhua Atlantic cod (Atlantic cod, No Restaurant cod) Cod, Icelandic Gadus morhua Atlantic cod (Atlantic cod, No Restaurant cod) Cod, Atlantic Gadus morhua Atlantic cod (Atlantic cod, No Restaurant cod) (food truck) Cod, Atlantic Gadus macrocephalus Pacific cod (Pacific cod, cod) Yes Restaurant Cod, Pacific Micromesistius australis Southern blue whiting Yes Restaurant (Southern blue whiting, Blue whiting, Blue cod) Cod, Norwegian Gadus morhua Atlantic cod (Atlantic cod, No Restaurant cod) Cod, Atlantic Gadus morhua Atlantic cod (Atlantic cod, No Restaurant cod) Cod, Atlantic Gadus morhua Atlantic cod (Atlantic cod, No Restaurant cod) Cod, Alaskan Gadus macrocephalus Pacific cod (Pacific cod, cod) No Grocery Store Cod, Pacific Gadus macrocephalus Pacific cod (Pacific cod, cod) No Grocery Store Cod, North Atlantic Gadus macrocephalus Pacific cod (Pacific cod, cod) Yes Restaurant Cod Gadus macrocephalus Pacific cod (Pacific cod, cod) No Grocery Store Cod, Icelandic Gadus morhua Atlantic cod (Atlantic cod, No Grocery cod) Store Cod, Icelandic Gadus morhua Atlantic cod (Atlantic cod, No Grocery cod) Store Euro Bass Gadus morhua Atlantic cod (Atlantic cod, Yes Restaurant cod) Grouper Epinephelus diacanthus Spinycheek grouper (n/a) Yes – E. -

Marine Fish Culture
FAU Institutional Repository http://purl.fcla.edu/fau/fauir This paper was submitted by the faculty of FAU’s Harbor Branch Oceanographic Institute. Notice: © 1998 Kluwer. This manuscript is an author version with the final publication available and may be cited as: Tucker, J. W., Jr. (1998). Marine fish culture. Boston: Kluwer Academic Publishers. MARINE FISH CULTURE by John W. Tucker, Jr., Ph.D. Harbor Branch Oceanographic Institution and Florida Institute of Technology, Melbourne KLUWER ACADEMIC PUBLISHERS Boston I Dordrecht I London Distributors for North, Central and South America: Kluwer Academic Publishers I 01 Philip Drive Assinippi Park Norwell, Massachusetts 02061 USA Telephone (781) 871-6600 Fax (781) 871-6528 E-Mail <[email protected]> Distributors for all other countries: Kluwer Academic Publishers Group Distribution Centre Post Office Box 322 3300 AH Dordrecht, THE NETHERLANDS Telephone 31 78 6392 392 Fax 31 78 6546 474 E-Mail <[email protected]> '' Electronic Services <http://www.wkap.nl> Library of Congress Cataloging-in-Publication Data Tucker, John W., 1948- Marine fish culture I by John W. Tucker, Jr. p. em. Includes bibliographical references (p. ) and index. ISBN 0-412-07151-7 (alk. paper) 1. Marine fishes. 2. Fish-culture. I. Title. SH163.T835 1998 639.3'2--dc21 98-42062 CIP Copyright © 1998 by Kluwer Academic Publishers All rights reserved. No part of this publication may be reproduced, stored in a retrieval system or transmitted in any form or by any means, mechanical, photo copying, recording, or otherwise, without the prior written permission of the publisher, Kluwer Academic Publishers, I 0 I Philip Drive, Assinippi Park, Norwell, Massachusetts 02061 Printed on acid-free paper. -

Evaluating Coexistence of Fish Species with Coastal Cutthroat Trout in Low Order Streams of Western Oregon and Washington, USA
fishes Article Evaluating Coexistence of Fish Species with Coastal Cutthroat Trout in Low Order Streams of Western Oregon and Washington, USA Kyle D. Martens 1,* and Jason Dunham 2 1 Washington Department of Natural Resources, 1111 Washington Street SE, Olympia, WA 98504, USA 2 U.S. Geological Survey, Forest and Rangeland Ecosystem Science Center, 3200 SW Jefferson Way, Corvallis, OR 97331, USA; [email protected] * Correspondence: [email protected] Abstract: When multiple species of fish coexist there are a host of potential ways through which they may interact, yet there is often a strong focus on studies of single species without considering these interactions. For example, many studies of forestry–stream interactions in the Pacific Northwest have focused solely on the most prevalent species: Coastal cutthroat trout. To examine the potential for interactions of other fishes with coastal cutthroat trout, we conducted an analysis of 281 sites in low order streams located on Washington’s Olympic Peninsula and along the central Oregon coast. Coastal cutthroat trout and juvenile coho salmon were the most commonly found salmonid species within these streams and exhibited positive associations with each other for both presence and density. Steelhead were negatively associated with the presence of coastal cutthroat trout as well as with coho salmon and sculpins (Cottidae). Coastal cutthroat trout most frequently shared streams with juvenile coho salmon. For densities of these co-occurring species, associations between these two species were relatively weak compared to the strong influences of physical stream conditions Citation: Martens, K.D.; Dunham, J. (size and gradient), suggesting that physical conditions may have more of an influence on density Evaluating Coexistence of Fish Species with Coastal Cutthroat Trout than species interactions. -

Lake Tahoe Fish Species
Description: o The Lohonton cutfhroot trout (LCT) is o member of the Solmonidqe {trout ond solmon) fomily, ond is thought to be omong the most endongered western solmonids. o The Lohonton cufihroot wos listed os endongered in 1970 ond reclossified os threotened in 1975. Dork olive bdcks ond reddish to yellow sides frequently chorocterize the LCT found in streoms. Steom dwellers reoch l0 inches in length ond only weigh obout I lb. Their life spon is less thon 5 yeors. ln streoms they ore opportunistic feeders, with diets consisting of drift orgonisms, typicolly terrestriol ond oquotic insects. The sides of loke-dwelling LCT ore often silvery. A brood, pinkish stripe moy be present. Historicolly loke dwellers reoched up to 50 inches in length ond weigh up to 40 pounds. Their life spon is 5-14yeors. ln lokes, smoll Lohontons feed on insects ond zooplonkton while lorger Lohonions feed on other fish. Body spots ore the diognostic chorocter thot distinguishes the Lohonion subspecies from the .l00 Poiute cutthroot. LCT typicolly hove 50 to or more lorge, roundish-block spots thot cover their entire bodies ond their bodies ore typicolly elongoted. o Like other cufihroot trout, they hove bosibronchiol teeth (on the bose of tongue), ond red sloshes under their iow (hence the nome "cutthroot"). o Femole sexuol moturity is reoch between oges of 3 ond 4, while moles moture ot 2 or 3 yeors of oge. o Generolly, they occur in cool flowing woier with ovoiloble cover of well-vegetoted ond stoble streom bonks, in oreos where there ore streom velocity breoks, ond in relotively silt free, rocky riffle-run oreos. -

History of Lahontan Cutthroat Trout in Spring Creek, Utah
Spring Creek Population History of the Pyramid Lake Rediscovery (Again) Unfortunately, given its small size, the trout Lahontan Cutthroat population at Spring Creek has a very low In October 2009, a team from Weber State probability of survival. It lacks the numbers The Lahontan cutthroat trout, Oncorhynchus University in conjunction with personnel and space necessary to maintain sufficient clarkii henshawi, is native to the Lahontan Basin from the DWR identified several specimens genetic diversity. It is believed that for a on the border between California and Nevada. believed to be of a pure or hybrid strain of mountain stream cutthroat population to For thousands of years it thrived and played the Pyramid Lake Lahontan cutthroat trout survive it must have a minimum of 3.3 km an important economic and cultural role in Spring Creek in Uintah, Utah. Using of habitat and an abundance in the area of among the Native American tribes of the electrofishers and dip nets, a 600 m stretch 0.3 fish per meter.3 Based on our region. The largest strain of this fish of the stream was sampled. A maximum observations, the Spring Creek population originated in Pyramid Lake, in western of 16 different individuals was collected in A Unique Environment has a maximum abundance of 0.1 fish/m Nevada and has reached recorded weights of two sampling trips. The fish appeared to Spring Creek’s unique vegetation and only 200 m of habitat. However, against up to 41 pounds, making it the largest “The Fish that Won’t Die” be restricted to a 200 m stretch. -

Rainbow Trout Oncorhynchus Mykiss
Rainbow Trout Oncorhynchus mykiss © Monterey Bay Aquarium USA Raceways and ponds Aquaculture Standard Version A2 February 6, 2017 Tyler Isaac, Seafood Watch Disclaimer Seafood Watch® strives to have all Seafood Reports reviewed for accuracy and completeness by external scientists with expertise in ecology, fisheries science and aquaculture. Scientific review, however, does not constitute an endorsement of the Seafood Watch® program or its recommendations on the part of the reviewing scientists. Seafood Watch® is solely responsible for the conclusions reached in this report. Final Seafood Recommendation Rainbow Trout Oncorhynchus mykiss United States (US) Raceways and ponds Criterion Score Rank Critical? C1 Data 8.18 GREEN C2 Effluent 8.00 GREEN NO C3 Habitat 9.33 GREEN NO C4 Chemicals 4.00 YELLOW NO C5 Feed 5.81 YELLOW NO C6 Escapes 7.00 GREEN NO C7 Disease 7.00 GREEN NO C8X Source 0.00 GREEN NO C9X Wildlife mortalities –2.00 GREEN NO C10X Introduced species escape –0.30 GREEN Total 47.02 Final score (0-10) 6.72 OVERALL RANKING Final Score 6.72 Initial rank GREEN Red criteria 0 Interim rank GREEN FINAL RANK Critical Criteria? NO GREEN Scoring note – scores range from zero to ten where zero indicates very poor performance and ten indicates the aquaculture operations have no significant impact. Summary The final numerical score for rainbow trout grown in raceways and ponds in the United States is 6.72. This numerical score is in the Green range, and with no Red criteria, the final ranking is Green and a recommendation of “Best Choice.” 2 Executive Summary Rainbow trout is native to many North American rivers and lakes that drain into the Pacific Ocean.