Changes in Growth and Physiological Parameters of ×Amarine Following an Exogenous Application of Gibberellic Acid and Methyl Jasmonate
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Summary of Offerings in the PBS Bulb Exchange, Dec 2012- Nov 2019
Summary of offerings in the PBS Bulb Exchange, Dec 2012- Nov 2019 3841 Number of items in BX 301 thru BX 463 1815 Number of unique text strings used as taxa 990 Taxa offered as bulbs 1056 Taxa offered as seeds 308 Number of genera This does not include the SXs. Top 20 Most Oft Listed: BULBS Times listed SEEDS Times listed Oxalis obtusa 53 Zephyranthes primulina 20 Oxalis flava 36 Rhodophiala bifida 14 Oxalis hirta 25 Habranthus tubispathus 13 Oxalis bowiei 22 Moraea villosa 13 Ferraria crispa 20 Veltheimia bracteata 13 Oxalis sp. 20 Clivia miniata 12 Oxalis purpurea 18 Zephyranthes drummondii 12 Lachenalia mutabilis 17 Zephyranthes reginae 11 Moraea sp. 17 Amaryllis belladonna 10 Amaryllis belladonna 14 Calochortus venustus 10 Oxalis luteola 14 Zephyranthes fosteri 10 Albuca sp. 13 Calochortus luteus 9 Moraea villosa 13 Crinum bulbispermum 9 Oxalis caprina 13 Habranthus robustus 9 Oxalis imbricata 12 Haemanthus albiflos 9 Oxalis namaquana 12 Nerine bowdenii 9 Oxalis engleriana 11 Cyclamen graecum 8 Oxalis melanosticta 'Ken Aslet'11 Fritillaria affinis 8 Moraea ciliata 10 Habranthus brachyandrus 8 Oxalis commutata 10 Zephyranthes 'Pink Beauty' 8 Summary of offerings in the PBS Bulb Exchange, Dec 2012- Nov 2019 Most taxa specify to species level. 34 taxa were listed as Genus sp. for bulbs 23 taxa were listed as Genus sp. for seeds 141 taxa were listed with quoted 'Variety' Top 20 Most often listed Genera BULBS SEEDS Genus N items BXs Genus N items BXs Oxalis 450 64 Zephyranthes 202 35 Lachenalia 125 47 Calochortus 94 15 Moraea 99 31 Moraea -

Boophone Disticha
Micropropagation and pharmacological evaluation of Boophone disticha Lee Cheesman Submitted in fulfilment of the academic requirements for the degree of Doctor of Philosophy Research Centre for Plant Growth and Development School of Life Sciences University of KwaZulu-Natal, Pietermaritzburg April 2013 COLLEGE OF AGRICULTURE, ENGINEERING AND SCIENCES DECLARATION 1 – PLAGIARISM I, LEE CHEESMAN Student Number: 203502173 declare that: 1. The research contained in this thesis, except where otherwise indicated, is my original research. 2. This thesis has not been submitted for any degree or examination at any other University. 3. This thesis does not contain other persons’ data, pictures, graphs or other information, unless specifically acknowledged as being sourced from other persons. 4. This thesis does not contain other persons’ writing, unless specifically acknowledged as being sourced from other researchers. Where other written sources have been quoted, then: a. Their words have been re-written but the general information attributed to them has been referenced. b. Where their exact words have been used, then their writing has been placed in italics and inside quotation marks, and referenced. 5. This thesis does not contain text, graphics or tables copied and pasted from the internet, unless specifically acknowledged, and the source being detailed in the thesis and in the reference section. Signed at………………………………....on the.....….. day of ……......……….2013 ______________________________ SIGNATURE i STUDENT DECLARATION Micropropagation and pharmacological evaluation of Boophone disticha I, LEE CHEESMAN Student Number: 203502173 declare that: 1. The research reported in this dissertation, except where otherwise indicated is the result of my own endeavours in the Research Centre for Plant Growth and Development, School of Life Sciences, University of KwaZulu-Natal, Pietermaritzburg. -

Clivia Miniata Colour Mutations and Their Breeding
Clivia Miniata Colour Mutations and their Breeding I don’t profess to be geneticists or to know very much about the way in which colour is inherited in Clivia, but by taking a very simple approach of line breeding and a lot of trial and error I have managed to produce true breeding lines of Clivia. This knowledge will be valuable to any breeder wishing to perpetuate a particular colour mutation. In the best interest of Clivia I have decided to write this article and attempt to share what I have learnt with others. It is my opinion that for a particular colour mutation to be inherited by sexual reproduction, a pathway has to occur i.e. the genes or other factors influencing the colour would have to line up. A simple way in which to view the chromosomes and the loci on which these chromosomes have mutated is by taking two rulers and placing them side by side, each representing different plants with similar colour mutations and only if both plants have mutated on the same point of the chromosomes (rulers) would the mutation be transferred to the off spring by sexual reproduction. Plants are relatively easy to line breed / inbreed because of the fact that they can be self- pollinated. Unfortunately, mechanisms are in place in plants that inhibit self-pollination and so more effort is required to perpetuate these colour mutations. The best-known colour mutation in Clivia is the yellow mutation occurring in Clivia miniata. Even here just because two plants are yellow doesn’t necessarily mean they have mutated at the same loci on the chromosome. -

STUDY on GROWTH, DEVELOPMENT and SOME BIOCHEMICAL ASPECTS of SEVERAL VARIETIES of Nerine
• STUDY ON GROWTH, DEVELOPMENT AND SOME BIOCHEMICAL ASPECTS OF SEVERAL VARIETIES OF Nerine by KUMALA DEWI partia,1 Submitted in,fulfilment of the requirements for the A degree of Master of Science Studies Department of Plant Science University of Tasmania May, 1993 DECLARATION To the best of my knowledge and belief, this thesis contains no material which has been submitted for the award of any other degree or diploma, nor does it contain any paraphrase of previously published material except where due reference is made in the text. Kumala Dewi ii ABSTRACT Nerine fothergillii bulbs were stored at different temperatures for a certain period of time and then planted and grown in an open condition. The effect of the different storage temperatures on carbohydrate -; content and endogenous gibberellins w Ct,5 examined in relation to flowering. Flowering percentage and flower number in each umbel was reduced when the bulbs were stored at 300 C while bulbs which received 50 C treatment possess earlier flowering and longer flower stalksthan bulbs without 5 0 C storage treatment. Carbohydrates in both outer and inner scales of N. fothergillii were examined semi-quantitatively by paper chromatography. Glucose, fructose and sucrose have been identified from paper chromatogramS. Endogenous gibberellins in N. fothergillii have been identified by GC - SIM and full mass spectra from GCMS. These include GA19, GA20 and G Al, their presence suggests the occurence of the early 13 - hydroxylation pathway. The response of N. bowdenii grown under Long Day (LD) and Short Day (SD) conditions w as studied. Ten plants from each treatment were examined at intervalsof 4 weeks. -

Complete Chloroplast Genomes Shed Light on Phylogenetic
www.nature.com/scientificreports OPEN Complete chloroplast genomes shed light on phylogenetic relationships, divergence time, and biogeography of Allioideae (Amaryllidaceae) Ju Namgung1,4, Hoang Dang Khoa Do1,2,4, Changkyun Kim1, Hyeok Jae Choi3 & Joo‑Hwan Kim1* Allioideae includes economically important bulb crops such as garlic, onion, leeks, and some ornamental plants in Amaryllidaceae. Here, we reported the complete chloroplast genome (cpDNA) sequences of 17 species of Allioideae, fve of Amaryllidoideae, and one of Agapanthoideae. These cpDNA sequences represent 80 protein‑coding, 30 tRNA, and four rRNA genes, and range from 151,808 to 159,998 bp in length. Loss and pseudogenization of multiple genes (i.e., rps2, infA, and rpl22) appear to have occurred multiple times during the evolution of Alloideae. Additionally, eight mutation hotspots, including rps15-ycf1, rps16-trnQ-UUG, petG-trnW-CCA , psbA upstream, rpl32- trnL-UAG , ycf1, rpl22, matK, and ndhF, were identifed in the studied Allium species. Additionally, we present the frst phylogenomic analysis among the four tribes of Allioideae based on 74 cpDNA coding regions of 21 species of Allioideae, fve species of Amaryllidoideae, one species of Agapanthoideae, and fve species representing selected members of Asparagales. Our molecular phylogenomic results strongly support the monophyly of Allioideae, which is sister to Amaryllioideae. Within Allioideae, Tulbaghieae was sister to Gilliesieae‑Leucocoryneae whereas Allieae was sister to the clade of Tulbaghieae‑ Gilliesieae‑Leucocoryneae. Molecular dating analyses revealed the crown age of Allioideae in the Eocene (40.1 mya) followed by diferentiation of Allieae in the early Miocene (21.3 mya). The split of Gilliesieae from Leucocoryneae was estimated at 16.5 mya. -

The Green Scene Brighten Winter with a Clivia Plant
Marion County Extension 210 N. Iowa St. Knoxville, IA 50138 641.842.2014 [email protected] February, 2019 Volume 17, Issue 2 The Green Scene Save a tree! Send your email address to [email protected] to receive this publication via e-mail. Brighten Winter with a Clivia Plant By Richard Jauon Flowers are one of the best antidotes to the icy winds of winter, and growing a houseplant that buds and blooms inside while all is dormant outside is particularly satisfying. This winter, as an alternative to the brightly blooming azaleas, chrysanthemums or traditional holiday plants, consider growing a clivia plant. What is a clivia? Clivia or kaffir lily (Clivia spp.) is a herbaceous flowering plant native to South Africa. Plants have long, arching, strap-like leaves (similar to an amaryllis) and produce dense clusters of trumpet-shaped flowers atop 18 to 24 inch stems. The flowers of Clivia minia- ta are typically orange with yellow eyes or centers. However, there are also several rare and expensive yellow-flowering cultivars. While clivias are not winter hardy in Iowa, they are excellent, low maintenance houseplants. How do I get a clivia to bloom? Clivias need a rest period of six to 12 weeks in fall and winter to initiate flower bud development. Temperatures dur- ing this time should be 40 to 55 degrees Fahrenheit. A guest bedroom, porch or a partially heated garage (temperatures must remain above 35 F) may be suitable plant locations. Water sparingly (about once a month). When flower stalks appear, move plants to a slightly warmer location and begin to water more frequently. -

Narcissus Pests
Bulletin 51 HMSO 13s Od [65p] net Narcissus Pests Ministry of Agriculture, Fisheries and Food MINISTRY OF AGRICULTURE, FISHERIES AND FOOD Narcissus Pests Bulletin 51 LONDON HER MAJESTY'S STATIONERY OFFICE 197o First published June 1932 Sixth edition 197o The Ministry does not accept responsibility for any of the private or trade advertisements included in this publication. SBN 11 240351 4 Foreword THE growers of Narcissus have been very fortunate in that the pests of this valuable crop have received specialist attention for nearly forty years. Names like W. E. H. Hodson and L. N. Staniland, both past authors of this Bulletin, rank high in the list of pioneer researchers on bulb pests in this country. They have been followed with no less enthusiasm by the con- tributors to this sixth edition which brings up-to-date our knowledge of the important pests of the crop and tested and practical methods of control. Although the present Bulletin mainly follows the pattern laid down by Mr. Hodson in 1932 many sections have been extensively rewritten. Mr. H. C. Woodville has dealt with narcissus flies as he did in 1958, and Mr. H. G. Morgan with detection of pests in the field, stem and other eelworms and their control. Mr. A. L. Winfield covered bulb scale mite and the general problem of hot-water treatment of bulbs, and chemical dips to control stem eelworm, and also contributed the notes on miscellaneous pests. Mr. P. Aitkenhead dealt with bulb mites. Mr. J. F. Southey provided the section dealing with eelworms as vectors of virus diseases of the crop. -

Van Zyverden's
Van Zyverden’s ALLIUM KARATAVIENSE Allium are in the same family as garlic, onions, chives and shallots. This makes gardeners wonder if they should include them in their ornamental gardening plans, as it conjures up images of supermarket produce. But because good garden designs are often made up of different shapes, allium’s rounded blooms make for high drama and interest in the garden. The Allium group gets more popular annually, from over 300 species to choose. They amaze everyone, and few plants create this kind of wow in the garden. We will be adding many new varieties shortly. Leaves and bulbs Commonly called Turkistan onion Deer and rodent resistant have a mild onion-like aroma when cut or bruised. About This Variety: Allium Karataviense is a compact, bulbous perennial that is ornamentally grown for both its foliage and its flowers. It is native to the Karatau Mountains (hence the specific epithet) in Kazakhstan. Broad-elliptic, spreading, gray-green, basal leaves appear in pairs. Leaves are sometimes mottled with purple. In late spring, a short but sturdy flowering stem rises from the center of each leaf pair. Each flowering stem is topped with a large spherical flower head containing tiny, star-shaped, dull pink florets. Flowers bloom in early summer. Flowers have a mild fragrance. Growing Instructions: As Alliums do not like wet feet, find a sunny location where the soil drains well or try to improve the drainage. The bulbs will rot in wet areas. Aside from that, almost no maintenance is required. Care Tip: Dig, divide, and replant bulbs after a few years of decreasing flower production. -

(Tribe Haemantheae) Inferred from Plastid and Nuclear Non-Coding DNA Sequences
Plant Syst. Evol. 244: 141–155 (2004) DOI 10.1007/s00606-003-0085-z Generic relationships among the baccate-fruited Amaryllidaceae (tribe Haemantheae) inferred from plastid and nuclear non-coding DNA sequences A. W. Meerow1, 2 and J. R. Clayton1 1 USDA-ARS-SHRS, National Germplasm Repository, Miami, Florida, USA 2 Fairchild Tropical Garden, Miami, Florida, USA Received October 22, 2002; accepted September 3, 2003 Published online: February 12, 2004 Ó Springer-Verlag 2004 Abstract. Using sequences from the plastid trnL-F Key words: Amaryllidaceae, Haemantheae, geo- region and nrDNA ITS, we investigated the phy- phytes, South Africa, monocotyledons, DNA, logeny of the fleshy-fruited African tribe Haeman- phylogenetics, systematics. theae of the Amaryllidaceae across 19 species representing all genera of the tribe. ITS and a Baccate fruits have evolved only once in the combined matrix produce the most resolute and Amaryllidaceae (Meerow et al. 1999), and well-supported tree with parsimony analysis. Two solely in Africa, but the genera possessing main clades are resolved, one comprising the them have not always been recognized as a monophyletic rhizomatous genera Clivia and Cryp- monophyletic group. Haemanthus L. and tostephanus, and a larger clade that unites Haemanthus and Scadoxus as sister genera to an Gethyllis L. were the first two genera of the Apodolirion/Gethyllis subclade. One of four group to be described (Linneaus 1753). Her- included Gethyllis species, G. lanuginosa, resolves bert (1837) placed Haemanthus (including as sister to Apodolirion with ITS. Relationships Scadoxus Raf.) and Clivia Lindl. in the tribe among the Clivia species are not in agreement with Amaryllidiformes, while Gethyllis was classi- a previous published phylogeny. -

Aksu-Zhabagly BIOSPHERE RESERVE National Commission Republic of Kazakhstan
Aksu-Zhabagly BIOSPHERE RESERVE National Commission Republic of Kazakhstan Kazakhstan National Committee Kazakhstan National Committee for the UNESCO Programme “Man and Biosphere” MAB, Institute of Zoology, 93 al-Farabi Str. Almaty, 050060 KAZAKHSTAN Kazakhstan National Committee Aksu-Zhabagly Biosphere Reserve NominatioN PART I: SUMMARY 1. PROPOSED NAME OF THE BIOSPHERE RESERVE: Aksu-Zhabagly Biosphere Reserve 2. COUNTRY: Kazakhstan Aksu-Zhabagly 4 FULFILLMENT OF THE THREE FUNCTIONS OF BIOSPHERE RESERVES 3. «Conservation — contribute to the conservation of landscapes, ecosystems, species and genetic variation» 3. 1 Aksu-Zhabagly biosphere reserve is located in the Western end of Talasskiy Alatau and Southern part of Karatau in the West Tien Shan. The whole region of the West Tien Shan is an Eastern outpost of Mediterranean atmospheric circulation, therefore it has a winter-spring rainfall. The mountain range of the West Tien Shan is a barrier that catches the moisture in the Western transport of air masses; in addition, this region is situated within the zone of the Southern deserts, where the annual temperature sum is high and about 4000-5000o C. As a result, this area is the most favorable for vegetation and preservation of many ancient relict species and plant communities. Moreover, the reserve’s ecosystems have a very close relationship with the natural systems of the Near East and the Mediterranean than to the rest of the ecosystems of the Tien Shan. The territory of Aksu Zhabagly has a high degree of representativeness at regional level. For example, it has almost all landscape types and sub-types of the West Tien Shan, except for deserts and gypsophilous subshrub communities, which are well below the reserve in altitude. -

Listado De Todas Las Plantas Que Tengo Fotografiadas Ordenado Por Familias Según El Sistema APG III (Última Actualización: 2 De Septiembre De 2021)
Listado de todas las plantas que tengo fotografiadas ordenado por familias según el sistema APG III (última actualización: 2 de Septiembre de 2021) GÉNERO Y ESPECIE FAMILIA SUBFAMILIA GÉNERO Y ESPECIE FAMILIA SUBFAMILIA Acanthus hungaricus Acanthaceae Acanthoideae Metarungia longistrobus Acanthaceae Acanthoideae Acanthus mollis Acanthaceae Acanthoideae Odontonema callistachyum Acanthaceae Acanthoideae Acanthus spinosus Acanthaceae Acanthoideae Odontonema cuspidatum Acanthaceae Acanthoideae Aphelandra flava Acanthaceae Acanthoideae Odontonema tubaeforme Acanthaceae Acanthoideae Aphelandra sinclairiana Acanthaceae Acanthoideae Pachystachys lutea Acanthaceae Acanthoideae Aphelandra squarrosa Acanthaceae Acanthoideae Pachystachys spicata Acanthaceae Acanthoideae Asystasia gangetica Acanthaceae Acanthoideae Peristrophe speciosa Acanthaceae Acanthoideae Barleria cristata Acanthaceae Acanthoideae Phaulopsis pulchella Acanthaceae Acanthoideae Barleria obtusa Acanthaceae Acanthoideae Pseuderanthemum carruthersii ‘Rubrum’ Acanthaceae Acanthoideae Barleria repens Acanthaceae Acanthoideae Pseuderanthemum carruthersii var. atropurpureum Acanthaceae Acanthoideae Brillantaisia lamium Acanthaceae Acanthoideae Pseuderanthemum carruthersii var. reticulatum Acanthaceae Acanthoideae Brillantaisia owariensis Acanthaceae Acanthoideae Pseuderanthemum laxiflorum Acanthaceae Acanthoideae Brillantaisia ulugurica Acanthaceae Acanthoideae Pseuderanthemum laxiflorum ‘Purple Dazzler’ Acanthaceae Acanthoideae Crossandra infundibuliformis Acanthaceae Acanthoideae Ruellia -
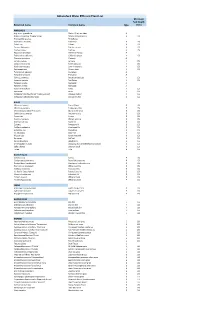
Abbotsford Water Efficient Plant List Minimum Soil Depth Botanical Name Common Name Type (Mm)
Abbotsford Water Efficient Plant List Minimum Soil Depth Botanical name Common name type (mm) ANNUALS Argemone grandifolia Statice/Sea Lavendar A Begonia x hybrida 'Dragon Wings' Dragon Wing begonia A 150 Bracteantha species Strawflower Calendula officinalis Calendula A 150 Coleus ssp. Coleus A 150 Cosmos bipinnatus Garden cosmos A 150 Cuphea llavea Cuphea A 150 Dyssocua tenuiloba Dahlberg Daisey A Eschscholzia californica California poppy A 150 Gazania spendens Gazania A Lantana camara Lantana A 150 Lobularia maritima Sweet alyssum A 150 Nigella damascena Love-in-the-mist A 150 Oesteopermum African daisy A 150 Pelargonium species Geranium A Portulaca oleracea Portulaca A Salvia guaranitica Anise-scented sage A 150 Scaevola aemula Fan flower A 150 Targetes erecta Marogold A Targetes erecta Marogold A Viola x wittrockiana Pansy A 150 Zinnia ssp. Zinnia A 150 Verbascum bombyciferum 'Arctic Summer' Broussa mullein A 150 Verbascum phlomoides 'Spica' Orange mullein A 150 BULBS Allium christophii Star of Persia B 150 Allium karataviense Turkestan onion B 150 Chionodoxa forbesii 'Pink Giant' Glory of the snow B 150 Colchicum autumnale Autumn crocus B 150 Crocus ssp. Crocus B 150 Eranthis hyemalis Winter aconite B 150 Erythronium ssp. Fawn lily B 150 Eucomis Pineapple lily B 150 Fritillaria meleagris Checkered lily B 150 Galanthus ssp. Snowdrop B 150 Iris reticulata Dwarf iris B 150 Muscari ssp. Grape hyacinth B 150 Narcissus Daffodil B 150 Nerine bowdenii Bowden lily B 150 Ornithogalum nutans Drooping Star of Bethlehem/Silverbells B 150 Scilla