The Mineralogical Ma Z:Ine
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Jahrbuch Der Kais. Kn. Geologischen Reichs-Anstalt
Digitised by the Harvard University, Download from The BHL http://www.biodiversitylibrary.org/; www.biologiezentrum.at Die Meteoritensammlung des k. k. mineralogischen Hof kabinetes in Wien am I. Mai 1885. Von Dr. Aristides Breziua. Mit vier Tafe'.n (Nr. 11— V). Von dieser Saminluug, welche schon zu Chladm's Zeiten von hohem wissenschaftlichen Werthe war und seither inamer eine erste Stelle einnahm, ist seit dem Jahre 1872 keine vollständige Gewichts- liste veröffentlicht worden ; in dem genannten Jahre gab der damalige Director des Kabinetes, Hofrath G. Tschermak, ein Verzeichnisse), das er bei seinem Abgange vom Museum durch einen Nachtrag ^) bis Ende September 1877 vervollständigte. Als mir nach dem Ausscheiden Tschermak's von meinem seither verstorbenen Vorstande, Hofrath Ritter v. Hochstetter, die Obsorge über die Sammlung übertragen wurde, war es mein nächstes Ziel, die Fälle aus den letzten Jahr- zehnten zu vervollständigen und die vielen nur durch kleine Splitter von einem Gramm und darunter vertretenen Localitäten durch grössere Stücke zu repräsentiren, weil so kleine Fragmente die petrographische Beschaffenheit eines gemengten Körpers nicht genügend erkennen lassen. Es zeigte sich bald, dass ein solches Ziel nur durch Anlegung einer eigenen Meteoritentauschsammlung zu erreichen war, welche bei Gele- genheit grösserer Fälle oder Funde mit Doublettenmateriale zu billigen Preisen versehen werden konnte und dann die Erwerbung auch der selteneren und kostbareren Fallorte auf dem Tauschwege gestattete; denn die Meteoritenpreise sind gegen frühere Jahrzehnte so wesentlich gestiegen, dass eine Ergänzung der Sammlung durch vorwiegenden Ankauf nicht mehr möglich ist, während andererseits eine Abgabe an- sehnlicher Stücke aus der Hauptsammlung, wie sie unter Hoernes- Haidinger üblich war, eine gewisse Beweglichkeit der Sammlung hervorbringt, welche bei einem so kostbaren Materiale wohl vermieden werden soll ; auch ein Tausch mit kleinen, von den Hauptstücken abge- kueipten Splittern, wie er ebenfalls häufig stattfand, bringt nur einen ') Die Meteoriten des k, k. -

James Hutton's Reputation Among Geologists in the Late Eighteenth and Nineteenth Centuries
The Geological Society of America Memoir 216 Revising the Revisions: James Hutton’s Reputation among Geologists in the Late Eighteenth and Nineteenth Centuries A. M. Celâl Şengör* İTÜ Avrasya Yerbilimleri Enstitüsü ve Maden Fakültesi, Jeoloji Bölümü, Ayazağa 34469 İstanbul, Turkey ABSTRACT A recent fad in the historiography of geology is to consider the Scottish polymath James Hutton’s Theory of the Earth the last of the “theories of the earth” genre of publications that had begun developing in the seventeenth century and to regard it as something behind the times already in the late eighteenth century and which was subsequently remembered only because some later geologists, particularly Hutton’s countryman Sir Archibald Geikie, found it convenient to represent it as a precursor of the prevailing opinions of the day. By contrast, the available documentation, pub- lished and unpublished, shows that Hutton’s theory was considered as something completely new by his contemporaries, very different from anything that preceded it, whether they agreed with him or not, and that it was widely discussed both in his own country and abroad—from St. Petersburg through Europe to New York. By the end of the third decade in the nineteenth century, many very respectable geologists began seeing in him “the father of modern geology” even before Sir Archibald was born (in 1835). Before long, even popular books on geology and general encyclopedias began spreading the same conviction. A review of the geological literature of the late eighteenth and the nineteenth centuries shows that Hutton was not only remembered, but his ideas were in fact considered part of the current science and discussed accord- ingly. -

Redacted for Privacy Roman A
AN ABSTRACT OF THE THESIS OF THOMAS WARD OSBORN IIIfor theMASTER OF SCIENCE (Name) (Degree) inCHEMISTRY presented on August 13, 1968 (Major) (Date) Title: SODIUM AND MANGANESE HOMOGENEITY INCHONDRITIC METEORITES Abstract approved:Redacted for Privacy Roman A. Schmitt Four to six one-gram specimens separated by adistance of several inches were obtained from each of 23 largechondritic meteorites (approximately 1 kg each) representing theolivine bronzite (H5), olivine hypersthene (L6) and enstatite (E5)classifications. Each specimen was analyzed for Na and Mn viainstrumental neutron activation analysis to a precision of about 1. 5% usingthe 2. 75 Mev and 0.84 Mev photopeaks for Na and Mn, respectively. The olivine bronzite (H5) falls were found to exhibit aMn homogeneity dispersion range of 3. 9% to 1. 7% for thelarge individual meteorites; Na dispersion range was 5. 2% to 4. 9%.One group of olivine bronzite (H5) finds consisting of five large meteoritefrag- ments exhibited a Mn dispersion range of 0. 8% to Z.7%; Na dispersion range was 1. 8% to 4. 7%.Another group of olivine bronzite (H5) finds consisting of four large pieces showed a Mn dispersion rangefrom 3. 8% to 17 % ; Na dispersion range,3. 8% to 31 % .The olivine hypersthene (L6) falls showed a Mn dispersion of 1.4% to 3.4%; Na dispersion, 2. 0% to 7. 1 %.The olivine hypersthene finds showed a Mn dispersion range of 2.5% to 5. 3%; Na dispersion range, 1.5% to 7. 6 %.The single enstatite find showed a. relative dispersion of 45% for Mn and 28% for Na. -

La Terre Est Ronde 2015-2016 Ok Mod 2016
ENSM-SE, 2° A : axe transverse P N 08/02/16 - 1 - ENSM-SE, 2° A : axe transverse P N 08/02/16 - 2 - ENSM-SE, 2° A : axe transverse P N 08/02/16 AVERTISSEMENT AU LECTEUR Aristarque de Samos Malgré plus de 10 années d’ajouts variés et de La dilapidation des ressources naturelles est, pour les modifications — et maintenant de pages web élèves Q- ou MC- nom de combustibles fossiles, irréversible pour la fraction déjà la page.htm, « Question » ou « Mot-Clef » indiqués au long de ce poly et consommée. Elle est même semble-t-il impossible à réduire que vous retrouverez en sur le site http://www.emse.fr/~bouchardon dans la pour les sociétés les moins industrialisées 2. Face à cette rubrique Wiki Elèves 2009-10-11 — ce poly de 200 pages, 23 situation, le plus dérangeant n’est pas tant la disparition tableaux et 310 figures environ n’est toujours pas un cours d’une énergie quand d’autres sont renouvelables et complet. Ce n’est d’ailleurs pas son objectif, même si sans seront largement à la disposition de l’homme dans le doute trouverez-vous ici ou là au long de ces cent cinquante futur, que l’épuisement d’un gisement source d’une foule pages, un fourmillement rebutant de connaissances parfois d’applications autres qu’énergétiques. Sur ce plan là, trop (?) pointues, typiques d’un cours. Le propos est de venir l’irréversibilité de nos actes doit peser dans nos décisions. en aide au lecteur, étudiant de l’EMSE ou autre, dans sa S’agissant d’éléments utiles à la fabrication de nos objets et compréhension des processus naturels en le baladant dans outils manufacturés, l’épuisement de la ressource est virtuel. -

Introduction to Cosmochemistry
Cambridge University Press 978-0-521-87862-3 - Cosmochemistry Harry Y. McSween and Gary R. Huss Excerpt More information 1 Introduction to cosmochemistry Overview Cosmochemistry is defined, and its relationship to geochemistry is explained. We describe the historical beginnings of cosmochemistry, and the lines of research that coalesced into the field of cosmochemistry are discussed. We then briefly introduce the tools of cosmochem- istry and the datasets that have been produced by these tools. The relationships between cosmochemistry and geochemistry, on the one hand, and astronomy, astrophysics, and geology, on the other, are considered. What is cosmochemistry? A significant portion of the universe is comprised of elements, ions, and the compounds formed by their combinations – in effect, chemistry on the grandest scale possible. These chemical components can occur as gases or superheated plasmas, less commonly as solids, and very rarely as liquids. Cosmochemistry is the study of the chemical composition of the universe and the processes that produced those compositions. This is a tall order, to be sure. Understandably, cosmo- chemistry focuses primarily on the objects in our own solar system, because that is where we have direct access to the most chemical information. That part of cosmochemistry encom- passes the compositions of the Sun, its retinue of planets and their satellites, the almost innumerable asteroids and comets, and the smaller samples (meteorites, interplanetary dust particles or “IDPs,” returned lunar samples) derived from them. From their chemistry, determined by laboratory measurements of samples or by various remote-sensing techniques, cosmochemists try to unravel the processes that formed or affected them and to fixthe chronology of these events. -

Meteoryt Meteoryt
BIULETYN MI£OŒNIKÓW METEORYTÓW METEORYTMETEORYT Nr 4 (40) Grudzieñ 2001 W numerze: Do kogo nale¿¹ meteoryty? Tagish Lake, Chassigny, Morasko, Gifhorn MARSMARS OZ£OCONYOZ£OCONY 4/2001 METEORYT 10 lat „Meteorytu”!str. 1 Od redaktora: Meteoryt (ISSN 1642-588X) – biuletyn dla mi³oœników mete- To wydanie „Meteorytu” nosi kolejny numer 40, co dla kwartalnika orytów wydawany przez Olsz- oznacza, ¿e koñczy siê 10 lat jego istnienia. Chcia³bym z tej okazji tyñskie Planetarium i Obserwa- podziêkowaæ najbardziej wytrwa³ym czytelnikom. Nie uda³o mi siê torium Astronomiczne, Muzeum odszukaæ pierwszej listy wysy³kowej, ale na drugiej, z 1993 roku Miko³aja Kopernika we From- widniej¹ nastêpuj¹ce osoby spoœród obecnych prenumeratorów: borku i Pallasite Press – wydaw- Jaros³aw Bandurowski, Janusz Bary³a, Tomasz Celeban, Leszek cê kwartalnika Meteorite, z któ- Chróst, Bartosz D¹browski, Jacek Dr¹¿kowski, Grzegorz Gnysiñski, rego pochodzi wiêksza czêœæ pu- Janusz Kosinski, Micha³ Kosmulski, Anna Kowarska, Jan blikowanych materia³ów. Kozakiewicz, Andrzej Manecki, Marek Micherdziñski, Marek Muciek, £ukasz Obroœlak, Micha³ Ostrowski, Dariusz Piasecki, Redaguje Andrzej S. Pilski Tadeusz Przylibski, Jerzy Puszcz, Krzysztof Socha, Katarzyna Sk³ad: Jacek Dr¹¿kowski Stanilewicz, Krzysztof Szczepaniuk, Marek Wierzchowiecki. Wtedy Druk: Jan, Lidzbark Warm. prenumeratorów by³o akurat 40. Dziêkujê, ¿e a¿ tylu wytrwa³o. Adres redakcji: Objêtoœæ tego numeru wzros³a do 44 stron, aby pomieœciæ skr. poczt. 6 wyczerpuj¹ce opracowanie na temat sytuacji prawnej meteorytów, 14-530 Frombork za które autorom bardzo dziêkujê. Niejednokrotnie styka³em siê tel. 0-55-243-7392 z pytaniem, do kogo nale¿y znaleziony meteoryt, i trudno by³o znaleŸæ e-mail: [email protected] na to odpowiedŸ. -

1 Introduction
Cambridge University Press 978-0-521-84035-4 - Atlas of Meteorites Monica M. Grady, Giovanni Pratesi and Vanni Moggi Cecchi Excerpt More information 1 Introduction Solar System history started some 4567 million years ago specimens collected by government-funded expeditions are with the collapse of an interstellar molecular cloud to a given a year–number combination with a prefix recording protoplanetary disk (the solar nebula) surrounding a central the icefield from which they were retrieved (e.g., Allan Hills star (the Sun). Evolution of the Solar System continued 84001), whereas meteorites collected in hot deserts are through a complex process of accretion, coagulation, simply numbered incrementally by region (e.g., Dar al Gani agglomeration, melting, differentiation and solidification, 262). The rules for naming newly recovered meteorites have followed by bombardment, collision, break-up, brecciation been standardized by the Nomenclature Committee of the and re-formation, then to varying extents by heating, meta- Meteoritical Society, which also assigns names to meteorites morphism, aqueous alteration and impact shock. One of the and keeps track of the total number of reported specimens. key goals of planetary science is to understand the primary This information is available at http://www./pi.usra.edu/ materials from which the Solar System formed, and how meteor/metbull.php. they have been modified as the Solar System evolved. The Newly recovered meteorites are also reported in the last two decades have seen a greater understanding of the Meteoritical Bulletin (published in the journal Meteoritics processes that led to the formation of the Sun and Solar and Planetary Sciences, and updated regularly on the web- System. -

United States National Museum
U. S. NATIONAL MUSEUM BULLETIN 149, FRONTISPIECE GEORGE PERKINS MERRILL BORN. MAY 31. 1854. DIED. AUGUST 15, 1929 SMITHSONIAN INSTITUTION UiNITED STATES NATIONAL MUSEUM Bulletin 149 COMPOSITION AND STRUCTURE OF METEORITES BY GEORGE P. MERRILL Head Curator of Geology, United States National Museum UNITED STATES GOVERNMENT PRINTING OFFICE WASHINGTON : 1930 the Superintendent of For »ale by Documents, Washington, D. C. ------- Price 40 i ADVERTISEMENT The scientific publications of the National Museum include two series, known, respectively, as Proceedings and Bulletin. The Proceedings, begun in 1878, is intended primarily as a medium for the publication of original papers, based on the collections of the National Museum, that set forth newly acquired facts in biology, anthropology, and geology, with descriptions of new forms and revi- sions of limited groups. Copies of each paper, in pamphlet form, are distributed as published to libraries and scientific organizations and to specialists and others interested in the different subjects. The dates at which these separate papers are published are recorded in the table of contents of each of the volumes. The Bulletins, the first of which was issued in 1875, consist of a series of separate publications comprising monographs of large zoological groups and other general systematic treatises (occasionally in several volumes), faunal works, reports of expeditions, catalogues of type-specimens, special collections, and other material of similar nature. The majority of the volumes are octavo in size, but a cpiarto size has been adopted in a few instances in which large plates were regarded as indispensable. In the Bulletin series appear volumes under the heading Contributions from the United States National Herbarium, in octavo form, published by the National Museum since 1902, which contain papers relating to the botanical collections of the Museum. -
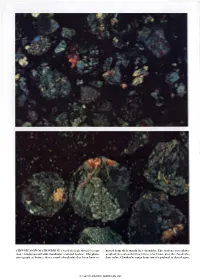
Chondrites and Chondrules
THIN SECTION OF CHONDRITE viewed through the microscope moved from their matrix in a chondrite. The sections were photo. (top) is interspersed with chondrules (colored bodies). The photo· graphed through polarizing filters, which here give the chondrules micrograph at bottom shows round chondrules that have been reo their color. Chondrules range from size of a pinhead to that of a pea. © 1963 SCIENTIFIC AMERICAN, INC Chondrites and Chondrules The first are stony m�eteorites; the second, the small spherical bodies they contain. There is evidence that the chondrules date back to the opening stages in the evolution of the solar system by John A. Wood n 1802 an English chemist named bodies occurring in chondrites soon came similar to those of the solar atmosphere. Edward C. Howard cautiously titled to be called chondrules. In 1930 the German spectroscopists I a paper he had written "Observa From the beginning investigators Ida and Walter Noddack pointed out tions on certain stony and metallic Sub have tended to believe that chondrites additional evidence. They found that stances, which at different Times are said are pieces of planetary matter in a very chondrites contain a more generous as to have fallen on the Earth." Howard primitive state. If this matter is not still sortment of trace elements in measur seems to have been the first person to in exactly the form it took when the able amounts than any type of earth examine carefully the internal structure planets first coalesced, it is not many rock does. In particular chondrites con of stony meteorites, and in all four speci evolutionary steps removed from that tain, mingled together, lithophile, chal mens he studied (stones from England, form. -

The History of Meteoritics - Overview
The history of meteoritics - overview G.J.H. McCALL 1, A.J. BOWDEN 2 & R.J. HOWARTH 3 144 Robert Franklin Way, South Cerney, Circencester, Gloucestershire GL7 5UD, UK (e-mail: joemccall @tiscali, co. uk) ZEarth and Physical Sciences, National Museums Liverpool, William Brown Street, Liverpool L3 8EN, UK (e-mail: Alan.Bowden@ liverpoolmuseums.org.uk) 3Department of Earth Sciences, University College London, Gower Street, London WC1E 6BT, UK (e-mail: [email protected]) Abstract: This volume was proposed after Peter Tandy and Joe McCall organized a 1-day meeting of the History of Geology Group, which is affiliated to the Geological Society, at the Natural History Museum in December 2003. This meeting covered the History of Meteori- tics up to 1920 and nine presentations were included, the keynote talk being given by Ursula Marvin. There was an enthusiastic audience of about 50, who expressed the view that this meeting should lead to a publication. Dr Cherry Lewis, the chairperson of the group, dis- cussed this with Joe McCall, who said that the material was too small for a Special Publi- cation, but it could be developed by expanding it, taking the history through the 20th century, when there was a revolution and immense expansion both in the scope of meteorite finds and the application of meteoritics to scientific research on a very broad front with the advent of the Space Age. This was agreed and a format of about 24 articles was designed, approaches being made to selected authors. The sections of this Special Publication relate to the early development of meteoritics as a science; collecting and museum collections; researches establishing the provenance of meteorites; and impact craters and tektites. -

The History of Meteoritics - Overview
Downloaded from http://sp.lyellcollection.org/ by guest on September 29, 2021 The history of meteoritics - overview G.J.H. McCALL 1, A.J. BOWDEN 2 & R.J. HOWARTH 3 144 Robert Franklin Way, South Cerney, Circencester, Gloucestershire GL7 5UD, UK (e-mail: joemccall @tiscali, co. uk) ZEarth and Physical Sciences, National Museums Liverpool, William Brown Street, Liverpool L3 8EN, UK (e-mail: Alan.Bowden@ liverpoolmuseums.org.uk) 3Department of Earth Sciences, University College London, Gower Street, London WC1E 6BT, UK (e-mail: [email protected]) Abstract: This volume was proposed after Peter Tandy and Joe McCall organized a 1-day meeting of the History of Geology Group, which is affiliated to the Geological Society, at the Natural History Museum in December 2003. This meeting covered the History of Meteori- tics up to 1920 and nine presentations were included, the keynote talk being given by Ursula Marvin. There was an enthusiastic audience of about 50, who expressed the view that this meeting should lead to a publication. Dr Cherry Lewis, the chairperson of the group, dis- cussed this with Joe McCall, who said that the material was too small for a Special Publi- cation, but it could be developed by expanding it, taking the history through the 20th century, when there was a revolution and immense expansion both in the scope of meteorite finds and the application of meteoritics to scientific research on a very broad front with the advent of the Space Age. This was agreed and a format of about 24 articles was designed, approaches being made to selected authors. -

Niobium and Tantalum III
Rediscovery of the Elements Niobium and Tantalum III James L. Marshall, Beta Eta 1971, and Virginia R. Marshall, Beta Eta 2003, Department of Chemistry, University of North Texas, Denton, TX 76203-5070, [email protected] Figure 1. Map of Berlin. The Apotheke zum weissen Schwan (Apothecary of the White Swan) no longer In the last issue of The HEXAGON 1j we exists; it was across the street from the present Heilige Geist Kirche (Church of the Holy Ghost), which still described how in 1809 William Hyde Wollaston stands today, Spandauer Straße 1, N52° 31.26 E13° 24.19. The Apotheke zum Bären (Apothecary of the (1766–1828) proclaimed 2 the two elements Bear) no longer exists; it was next to the present Nikolaikirche (Nicholas church), Probststraße - N52° columbium (known today as niobium), discov- 31.04 E13° 24.46. The Akadamiehaus (Old Berlin Akademie) was at present 28 Dorotheenstraße (original- ered in 1801 by Charles Hatchett (1765–1847), ly 7 Letzten Straße, then 10 Dorotheenstraße); now a parking garage - N52° 31.14 E13° 23.46. The and tantalum, discovered in 1802 by Anders Humboldt Universität zum Berlin (Berlin University) is located at Unter den Linden 6 - N52° 31.06 E13° Ekeberg (1767–1813), were identical. However, 23.63, with statues of Wilhelm Humboldt, founder of the university (located at “1”) and his brother there was a lingering suspicion among some Alexander Humboldt, the biogeological explorer (located at “2”). chemists that something was not quite right, because the densities of the source minerals eponymous Rose’s metal, a low-melting apothecary, and moved on to the Berlin columbite (from Connecticut) and tantalite (100°C) alloy of bismuth, tin, and lead.4a In Academy in 1800, becoming professor of the (from Finland) were different (5.918 and 7.953, 1771, Martin Heinrich Klaproth (1743–1817), University of Berlin when it was founded by the respectively).