Decoupled Jaws Promote Trophic Diversity in Cichlid Fishes
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Prosanta Chakrabarty
Prosanta Chakrabarty Museum of Natural Science, Dept. of Bio. Sci., 119 Foster Hall, Louisiana State University Baton Rouge, LA 70803 USA Office (225) 578-3079 | Fax: (225) 578-3075 e-mail: [email protected] webpage: http://www.prosanta.net EDUCATION 2006 Doctor of Philosophy in Ecology and Evolutionary Biology, University of Michigan, Dissertation: "Phylogenetic and Biogeographic Analyses of Greater Antillean and Middle American Cichlidae." 2000 Bachelor of Science in Applied Zoology, McGill University, Montréal, Québec. RECENT RESEARCH AND ADMINISTRATIVE POSITIONS 2014 – Present Associate Professor/Curator of Fishes, Louisiana State University, Department of Biological Sciences, Museum of Natural Science, LA. 2016 - Present Research Associate National Museum of Natural History Smithsonian, Washington, D.C. 2012 - Present Research Associate, Division of Vertebrate Zoology, American Museum of Natural History, NY. 2016 - 2017 Program Director for National Science Foundation (Visiting Scientist, Engineer Program), Systematics and Biodiversity Sciences Cluster in the Division of Environmental Biology, Directorate for Biological Sciences, VA. 2008 - 2014 Assistant Professor/Curator of Fishes, Louisiana State University, Department of Biological Sciences, Museum of Natural Science, LA. 2006 - 2008 Postdoctoral Fellow, American Museum of Natural History, Department of Ichthyology, NY. MAJOR GRANTS AND FELLOWSHIPS Over $2 million total as PI • NSF DEB: Collaborative Research: Not so Fast - Historical biogeography of freshwater fishes in Central America and the Greater Antilles, 2014-2019. • NSF IOS: Research Opportunity, 2018. • NSF: CSBR: Imminent and Critical Integration and Renovations to Herps and Fishes at the LSU Museum of Natural Science, 2016-2019. • National Academies Keck Futures Initiative: Crude Life: A Citizen Art and Science Investigation of Gulf of Mexico Biodiversity after the Deepwater Horizon Oil Spill, 2016-2018. -

Temporal Diversification of Mesoamerican Cichlid Fishes Across
MOLECULAR PHYLOGENETICS AND EVOLUTION Molecular Phylogenetics and Evolution 31 (2004) 754–764 www.elsevier.com/locate/ympev Temporal diversification of Mesoamerican cichlid fishes across a major biogeographic boundary C. Darrin Hulsey,a,* Francisco J. Garcıa de Leon, b Yara Sanchez Johnson,b Dean A. Hendrickson,c and Thomas J. Neara,1 a Center for Population Biology, Department of Evolution and Ecology, University of California-Davis, Davis, CA 95616, USA b Laboratorio de Biologıa Integrativa, Instituto Tecnologico de Cuidad Victoria (ITCV), Mexico c Section of Integrative Biology, University of Texas-Austin, Austin, TX 78712, USA Received 18 June 2003; revised 26 August 2003 Abstract The Mexican Neovolcanic Plateau sharply divides the vertebrate fauna of Mesoamerica where the climate of both the neotropics and temperate North America gradually blend. Only a few vertebrate groups such as the Heroine cichlids, distributed from South America to the Rio Grande in North America, are found both north and south of the Neovolcanic Plateau. To better understand the geography and temporal diversification of cichlids at this geologic boundary, we used mitochondrial DNA sequences of the cy- tochrome b (cyt b) gene to reconstruct the relationships of 52 of the approximately 80 species of Heroine cichlids in Mesoamerica. Our analysis suggests several cichlids in South America should be considered as part of the Mesoamerican Heroine clade because they and the cichlids north of the Isthmus of Panama are clearly supported as monophyletic with respect to all other Neotropical cichlids. We also recovered a group containing species in Paratheraps + Paraneetroplus + Vieja as the sister clade to Herichthys. Herichthys is the only cichlid clade north of the Mexican Plateau and it is monophyletic. -

Developmental Genetic Modularity of Cichlid Fish Dentitions
This is a repository copy of Biting into the Genome to Phenome Map: Developmental Genetic Modularity of Cichlid Fish Dentitions. White Rose Research Online URL for this paper: http://eprints.whiterose.ac.uk/109900/ Version: Accepted Version Article: Hulsey, C.D., Fraser, G.J. orcid.org/0000-0002-7376-0962 and Meyer, A. (2016) Biting into the Genome to Phenome Map: Developmental Genetic Modularity of Cichlid Fish Dentitions. Integrative and Comparative Biology, 56 (3). pp. 373-388. ISSN 1540-7063 https://doi.org/10.1093/icb/icw059 This is a pre-copyedited, author-produced version of an article accepted for publication in Integrative and Comparative Biology following peer review. The version of record Integr. Comp. Biol. (2016) 56 (3): 373-388 is available online at: https://doi.org/10.1093/icb/icw059. Reuse Unless indicated otherwise, fulltext items are protected by copyright with all rights reserved. The copyright exception in section 29 of the Copyright, Designs and Patents Act 1988 allows the making of a single copy solely for the purpose of non-commercial research or private study within the limits of fair dealing. The publisher or other rights-holder may allow further reproduction and re-use of this version - refer to the White Rose Research Online record for this item. Where records identify the publisher as the copyright holder, users can verify any specific terms of use on the publisher’s website. Takedown If you consider content in White Rose Research Online to be in breach of UK law, please notify us by emailing [email protected] including the URL of the record and the reason for the withdrawal request. -

Patterns of Evolution in Gobies (Teleostei: Gobiidae): a Multi-Scale Phylogenetic Investigation
PATTERNS OF EVOLUTION IN GOBIES (TELEOSTEI: GOBIIDAE): A MULTI-SCALE PHYLOGENETIC INVESTIGATION A Dissertation by LUKE MICHAEL TORNABENE BS, Hofstra University, 2007 MS, Texas A&M University-Corpus Christi, 2010 Submitted in Partial Fulfillment of the Requirements for the Degree of DOCTOR OF PHILOSOPHY in MARINE BIOLOGY Texas A&M University-Corpus Christi Corpus Christi, Texas December 2014 © Luke Michael Tornabene All Rights Reserved December 2014 PATTERNS OF EVOLUTION IN GOBIES (TELEOSTEI: GOBIIDAE): A MULTI-SCALE PHYLOGENETIC INVESTIGATION A Dissertation by LUKE MICHAEL TORNABENE This dissertation meets the standards for scope and quality of Texas A&M University-Corpus Christi and is hereby approved. Frank L. Pezold, PhD Chris Bird, PhD Chair Committee Member Kevin W. Conway, PhD James D. Hogan, PhD Committee Member Committee Member Lea-Der Chen, PhD Graduate Faculty Representative December 2014 ABSTRACT The family of fishes commonly known as gobies (Teleostei: Gobiidae) is one of the most diverse lineages of vertebrates in the world. With more than 1700 species of gobies spread among more than 200 genera, gobies are the most species-rich family of marine fishes. Gobies can be found in nearly every aquatic habitat on earth, and are often the most diverse and numerically abundant fishes in tropical and subtropical habitats, especially coral reefs. Their remarkable taxonomic, morphological and ecological diversity make them an ideal model group for studying the processes driving taxonomic and phenotypic diversification in aquatic vertebrates. Unfortunately the phylogenetic relationships of many groups of gobies are poorly resolved, obscuring our understanding of the evolution of their ecological diversity. This dissertation is a multi-scale phylogenetic study that aims to clarify phylogenetic relationships across the Gobiidae and demonstrate the utility of this family for studies of macroevolution and speciation at multiple evolutionary timescales. -

Estado Actual De La Arqueozoología Latinoamericana Current Advances
Estado Actual de la Arqueozoología Latinoamericana Current Advances for the Latin-American Archaeozoology Guillermo Mengoni Goñalons Joaquín Arroyo-Cabrales Óscar J. Polaco Felisa J. Aguilar Editores INSTITUTO NACIONAL DE ANTROPOLOGÍA E HISTORIA CONSEJO NACIONAL DE CIENCIA Y TECNOLOGÍA INTERNATIONAL COUNCIL FOR ARCHAEOZOOLOGY UNIVERSIDAD DE BUENOS AIRES Estado actual de la arqueozoologia latinoamericana = Current advances for the Latin-American archaeozoology / editores Guillermo Mengoni Goñalons... [et al.]. – México: Instituto Nacional de Antropología e Historia: Consejo Nacional de Ciencia y Tecnología: International Council for Archaeozoology: Universidad de Buenos Aires, 2010. 190 p.: il. ; 28 cm. ISBN: 978-607-484-145-9 Item publicado In Memoriam de Óscar J. Polaco Ramos. 1. Arqueozoología – Latinoamérica – Congresos. 2. Paleozoología – Latinoamérica – Congresos. 3. Animales prehistóricos – Latinoamérica – Congresos. 4. Fósiles – Latinoamérica – Congresos. I. Mengoni Goñalons, Guillermo, ed. II. Arroyo Cabrales, Joaquín, ed. III. Polaco Ramos, Óscar J., 1952-2009. IV. Aguilar, Felisa J., ed. V. t. LC: CC79.5 / A5 / E877 Primera edición: 2010 DR © Instituto Nacional de Antropología e Historia. Córdoba 45, Col. Roma, C. P. 06700, México, D. F. ISBN: 978-607-484-145-9 Todos los derechos reservados. Queda prohibida la reproducción total o parcial de esta obra por cualquier medio o procedimiento, comprendidos la reprografía y el tratamiento informático, la fotocopia o la grabación sin la previa autorización por escrito de los titulares de los derechos de esta edición. Impreso en México / Printed in Mexico RQUEOZOOLOGÍA EN LA AJA MÉRICA ENTRAL A B A C Richard Cooke1,2 ICARAGUA OSTA ICA Y ANAMÁ Juan Guillermo Martín (N , C R P ) Rincón2 1. Smithsonian Tropical Research Institute (Panamá) 2. -

History of Fishes - Structural Patterns and Trends in Diversification
History of fishes - Structural Patterns and Trends in Diversification AGNATHANS = Jawless • Class – Pteraspidomorphi • Class – Myxini?? (living) • Class – Cephalaspidomorphi – Osteostraci – Anaspidiformes – Petromyzontiformes (living) Major Groups of Agnathans • 1. Osteostracida 2. Anaspida 3. Pteraspidomorphida • Hagfish and Lamprey = traditionally together in cyclostomata Jaws = GNATHOSTOMES • Gnathostomes: the jawed fishes -good evidence for gnathostome monophyly. • 4 major groups of jawed vertebrates: Extinct Acanthodii and Placodermi (know) Living Chondrichthyes and Osteichthyes • Living Chondrichthyans - usually divided into Selachii or Elasmobranchi (sharks and rays) and Holocephali (chimeroids). • • Living Osteichthyans commonly regarded as forming two major groups ‑ – Actinopterygii – Ray finned fish – Sarcopterygii (coelacanths, lungfish, Tetrapods). • SARCOPTERYGII = Coelacanths + (Dipnoi = Lung-fish) + Rhipidistian (Osteolepimorphi) = Tetrapod Ancestors (Eusthenopteron) Close to tetrapods Lungfish - Dipnoi • Three genera, Africa+Australian+South American ACTINOPTERYGII Bichirs – Cladistia = POLYPTERIFORMES Notable exception = Cladistia – Polypterus (bichirs) - Represented by 10 FW species - tropical Africa and one species - Erpetoichthys calabaricus – reedfish. Highly aberrant Cladistia - numerous uniquely derived features – long, independent evolution: – Strange dorsal finlets, Series spiracular ossicles, Peculiar urohyal bone and parasphenoid • But retain # primitive Actinopterygian features = heavy ganoid scales (external -
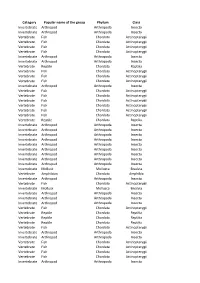
Category Popular Name of the Group Phylum Class Invertebrate
Category Popular name of the group Phylum Class Invertebrate Arthropod Arthropoda Insecta Invertebrate Arthropod Arthropoda Insecta Vertebrate Fish Chordata Actinopterygii Vertebrate Fish Chordata Actinopterygii Vertebrate Fish Chordata Actinopterygii Vertebrate Fish Chordata Actinopterygii Invertebrate Arthropod Arthropoda Insecta Invertebrate Arthropod Arthropoda Insecta Vertebrate Reptile Chordata Reptilia Vertebrate Fish Chordata Actinopterygii Vertebrate Fish Chordata Actinopterygii Vertebrate Fish Chordata Actinopterygii Invertebrate Arthropod Arthropoda Insecta Vertebrate Fish Chordata Actinopterygii Vertebrate Fish Chordata Actinopterygii Vertebrate Fish Chordata Actinopterygii Vertebrate Fish Chordata Actinopterygii Vertebrate Fish Chordata Actinopterygii Vertebrate Fish Chordata Actinopterygii Vertebrate Reptile Chordata Reptilia Invertebrate Arthropod Arthropoda Insecta Invertebrate Arthropod Arthropoda Insecta Invertebrate Arthropod Arthropoda Insecta Invertebrate Arthropod Arthropoda Insecta Invertebrate Arthropod Arthropoda Insecta Invertebrate Arthropod Arthropoda Insecta Invertebrate Arthropod Arthropoda Insecta Invertebrate Arthropod Arthropoda Insecta Invertebrate Arthropod Arthropoda Insecta Invertebrate Mollusk Mollusca Bivalvia Vertebrate Amphibian Chordata Amphibia Invertebrate Arthropod Arthropoda Insecta Vertebrate Fish Chordata Actinopterygii Invertebrate Mollusk Mollusca Bivalvia Invertebrate Arthropod Arthropoda Insecta Invertebrate Arthropod Arthropoda Insecta Invertebrate Arthropod Arthropoda Insecta Vertebrate -

Abstracts Part 1
375 Poster Session I, Event Center – The Snowbird Center, Friday 26 July 2019 Maria Sabando1, Yannis Papastamatiou1, Guillaume Rieucau2, Darcy Bradley3, Jennifer Caselle3 1Florida International University, Miami, FL, USA, 2Louisiana Universities Marine Consortium, Chauvin, LA, USA, 3University of California, Santa Barbara, Santa Barbara, CA, USA Reef Shark Behavioral Interactions are Habitat Specific Dominance hierarchies and competitive behaviors have been studied in several species of animals that includes mammals, birds, amphibians, and fish. Competition and distribution model predictions vary based on dominance hierarchies, but most assume differences in dominance are constant across habitats. More recent evidence suggests dominance and competitive advantages may vary based on habitat. We quantified dominance interactions between two species of sharks Carcharhinus amblyrhynchos and Carcharhinus melanopterus, across two different habitats, fore reef and back reef, at a remote Pacific atoll. We used Baited Remote Underwater Video (BRUV) to observe dominance behaviors and quantified the number of aggressive interactions or bites to the BRUVs from either species, both separately and in the presence of one another. Blacktip reef sharks were the most abundant species in either habitat, and there was significant negative correlation between their relative abundance, bites on BRUVs, and the number of grey reef sharks. Although this trend was found in both habitats, the decline in blacktip abundance with grey reef shark presence was far more pronounced in fore reef habitats. We show that the presence of one shark species may limit the feeding opportunities of another, but the extent of this relationship is habitat specific. Future competition models should consider habitat-specific dominance or competitive interactions. -

Cytogenetics of Gymnogeophagus Setequedas (Cichlidae: Geophaginae), with Comments on Its Geographical Distribution
Neotropical Ichthyology, 15(2): e160035, 2017 Journal homepage: www.scielo.br/ni DOI: 10.1590/1982-0224-20160035 Published online: 26 June 2017 (ISSN 1982-0224) Copyright © 2017 Sociedade Brasileira de Ictiologia Printed: 30 June 2017 (ISSN 1679-6225) Cytogenetics of Gymnogeophagus setequedas (Cichlidae: Geophaginae), with comments on its geographical distribution Leonardo M. Paiz1, Lucas Baumgärtner2, Weferson J. da Graça1,3, Vladimir P. Margarido1,2 and Carla S. Pavanelli1,3 We provide cytogenetic data for the threatened species Gymnogeophagus setequedas, and the first record of that species collected in the Iguaçu River, within the Iguaçu National Park’s area of environmental preservation, which is an unexpected occurrence for that species. We verified a diploid number of 2n = 48 chromosomes (4sm + 24st + 20a) and the presence of heterochromatin in centromeric and pericentromeric regions, which are conserved characters in the Geophagini. The multiple nucleolar organizer regions observed in G. setequedas are considered to be apomorphic characters in the Geophagini, whereas the simple 5S rDNA cistrons located interstitially on the long arm of subtelocentric chromosomes represent a plesiomorphic character. Because G. setequedas is a threatened species that occurs in lotic waters, we recommend the maintenance of undammed environments within its known area of distribution. Keywords: Chromosomes, Conservation, Iguaçu River, Karyotype, Paraná River. Fornecemos dados citogenéticos para a espécie ameaçada Gymnogeophagus setequedas, e o primeiro registro da espécie coletado no rio Iguaçu, na área de preservação ambiental do Parque Nacional do Iguaçu, a qual é uma área de ocorrência inesperada para esta espécie. Verificamos em G. setequedas 2n = 48 cromossomos (4sm + 24st + 20a) e heterocromatina presente nas regiões centroméricas e pericentroméricas, as quais indicam caracteres conservados em Geophagini. -

2010 Board of Governors Report
American Society of Ichthyologists and Herpetologists Board of Governors Meeting Westin – Narragansett Ballroom B Providence, Rhode Island 7 July 2010 Maureen A. Donnelly Secretary Florida International University College of Arts & Sciences 11200 SW 8th St. - ECS 450 Miami, FL 33199 [email protected] 305.348.1235 13 June 2010 The ASIH Board of Governor's is scheduled to meet on Wednesday, 7 July 2010 from 5:00 – 7:00 pm in the Westin Hotel in Narragansett Ballroom B. President Hanken plans to move blanket acceptance of all reports included in this book that cover society business for 2009 and 2010 (in part). The book includes the ballot information for the 2010 elections (Board of Governors and Annual Business Meeting). Governors can ask to have items exempted from blanket approval. These exempted items will be acted upon individually. We will also act individually on items exempted by the Executive Committee. Please remember to bring this booklet with you to the meeting. I will bring a few extra copies to Providence. Please contact me directly (email is best - [email protected]) with any questions you may have. Please notify me if you will not be able to attend the meeting so I can share your regrets with the Governors. I will leave for Providence (via Boston on 4 July 2010) so try to contact me before that date if possible. I will arrive in Providence on the afternoon of 6 July 2010 The Annual Business Meeting will be held on Sunday 11 July 2010 from 6:00 to 8:00 pm in The Rhode Island Convention Center (RICC) in Room 556 AB. -

Comparative Cytogenetics of Neotropical Cichlid Fishes
COMPARATIVE A peer-reviewed open-access journal CompCytogen 8(3): 169–183 (2014)Comparative cytogenetics of Neotropical cichlid fishes... 169 doi: 10.3897/CompCytogen.v8i3.7279 RESEARCH ARTICLE Cytogenetics www.pensoft.net/journals/compcytogen International Journal of Plant & Animal Cytogenetics, Karyosystematics, and Molecular Systematics Comparative cytogenetics of Neotropical cichlid fishes (Nannacara, Ivanacara and Cleithracara) indicates evolutionary reduction of diploid chromosome numbers Lucie Hodaňová1, Lukáš Kalous1, Zuzana Musilová1,2,3 1 Department of Zoology and Fisheries, Faculty of Agrobiology, Food and Natural Resources, Czech University of Life Sciences Prague, Prague, Czech Republic 2 Laboratory of Fish Genetics, Institute of Animal Physiology and Genetics AV CR, Libechov, Czech Republic 3 Zoological Institute, University of Basel, Switzerland Corresponding author: Zuzana Musilová ([email protected]) Academic editor: Petr Rab | Received 17 February 2014 | Accepted 29 July 2014 | Published 8 August 2014 http://zoobank.org/E973BC3C-DBEA-4915-9E63-6BBEE9E0940D Citation: Hodaňová L, Kalous L, Musilová Z (2014) Comparative cytogenetics of Neotropical cichlid fishes Nannacara( , Ivanacara and Cleithracara) indicates evolutionary reduction of diploid chromosome numbers. Comparative Cytogenetics 8(3): 169–183. doi: 10.3897/CompCytogen.v8i3.7279 Abstract A comparative cytogenetic analysis was carried out in five species of a monophyletic clade of neotropical Cichlasomatine cichlids, namely Cleithracara maronii Steindachner, 1881, Ivanacara adoketa (Kullander & Prada-Pedreros, 1993), Nannacara anomala Regan, 1905, N. aureocephalus Allgayer, 1983 and N. tae- nia Regan, 1912. Karyotypes and other chromosomal characteristics were revealed by CDD banding and mapped onto the phylogenetic hypothesis based on molecular analyses of four genes, namely cyt b, 16S rRNA, S7 and RAG1. The diploid numbers of chromosomes ranged from 44 to 50, karyotypes were com- posed predominantly of monoarmed chromosomes and one to three pairs of CMA3 signal were observed. -

Advances in Fish Biology Symposium,” We Are Including 48 Oral and Poster Papers on a Diverse Range of Species, Covering a Number of Topics
Advances in Fish Biology SYMPOSIUM PROCEEDINGS Adalberto Val Don MacKinlay International Congress on the Biology of Fish Tropical Hotel Resort, Manaus Brazil, August 1-5, 2004 Copyright © 2004 Physiology Section, American Fisheries Society All rights reserved International Standard Book Number(ISBN) 1-894337-44-1 Notice This publication is made up of a combination of extended abstracts and full papers, submitted by the authors without peer review. The formatting has been edited but the content is the responsibility of the authors. The papers in this volume should not be cited as primary literature. The Physiology Section of the American Fisheries Society offers this compilation of papers in the interests of information exchange only, and makes no claim as to the validity of the conclusions or recommendations presented in the papers. For copies of these Symposium Proceedings, or the other 20 Proceedings in the Congress series, contact: Don MacKinlay, SEP DFO, 401 Burrard St Vancouver BC V6C 3S4 Canada Phone: 604-666-3520 Fax 604-666-0417 E-mail: [email protected] Website: www.fishbiologycongress.org ii PREFACE Fish are so important in our lives that they have been used in thousands of different laboratories worldwide to understand and protect our environment; to understand and ascertain the foundation of vertebrate evolution; to understand and recount the history of vertebrate colonization of isolated pristine environments; and to understand the adaptive mechanisms to extreme environmental conditions. More importantly, fish are one of the most important sources of protein for the human kind. Efforts at all levels have been made to increase fish production and, undoubtedly, the biology of fish, especially the biology of unknown species, has much to contribute.