Modeling Malaria Cases Associated with Environmental Risk Factors in Ethiopia Using Geographically Weighted Regression
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

An Analysis of the Afar-Somali Conflict in Ethiopia and Djibouti
Regional Dynamics of Inter-ethnic Conflicts in the Horn of Africa: An Analysis of the Afar-Somali Conflict in Ethiopia and Djibouti DISSERTATION ZUR ERLANGUNG DER GRADES DES DOKTORS DER PHILOSOPHIE DER UNIVERSTÄT HAMBURG VORGELEGT VON YASIN MOHAMMED YASIN from Assab, Ethiopia HAMBURG 2010 ii Regional Dynamics of Inter-ethnic Conflicts in the Horn of Africa: An Analysis of the Afar-Somali Conflict in Ethiopia and Djibouti by Yasin Mohammed Yasin Submitted in partial fulfilment of the requirements for the degree PHILOSOPHIAE DOCTOR (POLITICAL SCIENCE) in the FACULITY OF BUSINESS, ECONOMICS AND SOCIAL SCIENCES at the UNIVERSITY OF HAMBURG Supervisors Prof. Dr. Cord Jakobeit Prof. Dr. Rainer Tetzlaff HAMBURG 15 December 2010 iii Acknowledgments First and foremost, I would like to thank my doctoral fathers Prof. Dr. Cord Jakobeit and Prof. Dr. Rainer Tetzlaff for their critical comments and kindly encouragement that made it possible for me to complete this PhD project. Particularly, Prof. Jakobeit’s invaluable assistance whenever I needed and his academic follow-up enabled me to carry out the work successfully. I therefore ask Prof. Dr. Cord Jakobeit to accept my sincere thanks. I am also grateful to Prof. Dr. Klaus Mummenhoff and the association, Verein zur Förderung äthiopischer Schüler und Studenten e. V., Osnabruck , for the enthusiastic morale and financial support offered to me in my stay in Hamburg as well as during routine travels between Addis and Hamburg. I also owe much to Dr. Wolbert Smidt for his friendly and academic guidance throughout the research and writing of this dissertation. Special thanks are reserved to the Department of Social Sciences at the University of Hamburg and the German Institute for Global and Area Studies (GIGA) that provided me comfortable environment during my research work in Hamburg. -

Districts of Ethiopia
Region District or Woredas Zone Remarks Afar Region Argobba Special Woreda -- Independent district/woredas Afar Region Afambo Zone 1 (Awsi Rasu) Afar Region Asayita Zone 1 (Awsi Rasu) Afar Region Chifra Zone 1 (Awsi Rasu) Afar Region Dubti Zone 1 (Awsi Rasu) Afar Region Elidar Zone 1 (Awsi Rasu) Afar Region Kori Zone 1 (Awsi Rasu) Afar Region Mille Zone 1 (Awsi Rasu) Afar Region Abala Zone 2 (Kilbet Rasu) Afar Region Afdera Zone 2 (Kilbet Rasu) Afar Region Berhale Zone 2 (Kilbet Rasu) Afar Region Dallol Zone 2 (Kilbet Rasu) Afar Region Erebti Zone 2 (Kilbet Rasu) Afar Region Koneba Zone 2 (Kilbet Rasu) Afar Region Megale Zone 2 (Kilbet Rasu) Afar Region Amibara Zone 3 (Gabi Rasu) Afar Region Awash Fentale Zone 3 (Gabi Rasu) Afar Region Bure Mudaytu Zone 3 (Gabi Rasu) Afar Region Dulecha Zone 3 (Gabi Rasu) Afar Region Gewane Zone 3 (Gabi Rasu) Afar Region Aura Zone 4 (Fantena Rasu) Afar Region Ewa Zone 4 (Fantena Rasu) Afar Region Gulina Zone 4 (Fantena Rasu) Afar Region Teru Zone 4 (Fantena Rasu) Afar Region Yalo Zone 4 (Fantena Rasu) Afar Region Dalifage (formerly known as Artuma) Zone 5 (Hari Rasu) Afar Region Dewe Zone 5 (Hari Rasu) Afar Region Hadele Ele (formerly known as Fursi) Zone 5 (Hari Rasu) Afar Region Simurobi Gele'alo Zone 5 (Hari Rasu) Afar Region Telalak Zone 5 (Hari Rasu) Amhara Region Achefer -- Defunct district/woredas Amhara Region Angolalla Terana Asagirt -- Defunct district/woredas Amhara Region Artuma Fursina Jile -- Defunct district/woredas Amhara Region Banja -- Defunct district/woredas Amhara Region Belessa -- -

The Case of Smallholder Farmers at Guba Lafto Woreda, Amhara National Region State, Ethiopia
VULNERABILITY AND ADAPTATION PRACTICES TO CLIMATE CHANGE: THE CASE OF SMALLHOLDER FARMERS AT GUBA LAFTO WOREDA, AMHARA NATIONAL REGION STATE, ETHIOPIA M.Sc. THESIS TSEGAYE TEMESGEN WONDO GENET COLLEGE OF FORESTRY AND NATURAL RESOURCES, HAWASSA UNIVERSITY, ETHIOPIA JUNE, 2018 VULNERABILITY AND ADAPTATION PRACTICES TO CLIMATE CHANGE: THE CASE OF SMALLHOLDER FARMERS AT GUBA LAFTO WOREDA, AMHARA NATIONAL REGION STATE, ETHIOPIA TSEGAYE TEMESGEN A THESIS SUBMITTED TO THE DEPARTMENT OF CLIMATE SMART AGRICULTURAL LANDSCAPE ASSESMENT, WONDO GENET COLLEGE OF FORESTRY AND NATURAL RESOURCES, SCHOOL OF GRADUATE STUDIES WONDO GENET COLLEGE OF FORESTRY AND NATURAL RESOURCES WONDO GENET, ETHIOPIA IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF MASTER OF SCIENCE IN CLIMATE SMART AGRICULTURAL LANDSCAPE ASSESSMENT (SPECIALIZATION: CLIMATE SMART AGRICULTURAL LANDSCAPE ASSESMENT) JUNE, 2018 II Approval sheet1 This is to certify that the thesis entitled “vulnerability and adaptation practices to climate change: the case of smallholder farmers at Guba Lafto woreda, Amhara national region state, Ethiopia” is submitted in partial fulfillment of the requirement for the degree of Master of Sciences with specialization in climate smart agricultural landscape assessment. It is a record of original research carried out by Tsegaye Temesgen Id. No Msc/CSAL/R0012/09, under my supervision; and no part of the thesis has been submitted for any other degree or diploma. The assistance and help received during the courses of this investigation have been duly acknowledged. -

Background Information Study Tour Ethiopia 2007
Landscape Transformation and Sustainable Development in Ethiopia | downloaded: 13.3.2017 Background information for a study tour through Ethiopia, 4-20 September 2006 University of Bern Institute of Geography https://doi.org/10.7892/boris.71076 2007 source: Cover photographs Left: Digging an irrigation channel near Lake Maybar to substitute missing rain in the drought of 1984/1985. Hans Hurni, 1985. Centre: View of the Simen Mountains from the lowlands in the Simen Mountains National Park. Gudrun Schwilch, 1994. Right: Extreme soil degradation in the Andit Tid area, a research site of the Soil Conservation Research Programme (SCRP). Hans Hurni, 1983. Landscape Transformation and Sustainable Development in Ethiopia Background information for a study tour through Ethiopia, 4-20 September 2006 University of Bern Institute of Geography 2007 3 Impressum © 2007 University of Bern, Institute of Geography, Centre for Development and Environment Concept: Hans Hurni Coordination and layout: Brigitte Portner Contributors: Alemayehu Assefa, Amare Bantider, Berhan Asmamew, Manuela Born, Antonia Eisenhut, Veronika Elgart, Elias Fekade, Franziska Grossenbacher, Christine Hauert, Karl Herweg, Hans Hurni, Kaspar Hurni, Daniel Loppacher, Sylvia Lörcher, Eva Ludi, Melese Tesfaye, Andreas Obrecht, Brigitte Portner, Eduardo Ronc, Lorenz Roten, Michael Rüegsegger, Stefan Salzmann, Solomon Hishe, Ivo Strahm, Andres Strebel, Gianreto Stuppani, Tadele Amare, Tewodros Assefa, Stefan Zingg. Citation: Hurni, H., Amare Bantider, Herweg, K., Portner, B. and H. Veit (eds.). 2007. Landscape Transformation ansd Sustainable Development in Ethiopia. Background information for a study tour through Ethiopia, 4-20 September 2006, compiled by the participants. Centre for Development and Environment, University of Bern, Bern, 321 pp. Available from: www.cde.unibe.ch. -

20210714 Access Snapshot- Tigray Region June 2021 V2
ETHIOPIA Tigray: Humanitarian Access Snapshot (July 2021) As of 31 July 2021 The conflict in Tigray continues despite the unilateral ceasefire announced by the Ethiopian Federal Government on 28 June, which resulted in the withdrawal of the Ethiopian National Overview of reported incidents July Since Nov July Since Nov Defense Forces (ENDF) and Eritrea’s Defense Forces (ErDF) from Tigray. In July, Tigray forces (TF) engaged in a military offensive in boundary areas of Amhara and Afar ERITREA 13 153 2 14 regions, displacing thousands of people and impacting access into the area. #Incidents impacting Aid workers killed Federal authorities announced the mobilization of armed forces from other regions. The Amhara region the security of aid Tahtay North workers Special Forces (ASF), backed by ENDF, maintain control of Western zone, with reports of a military Adiyabo Setit Humera Western build-up on both sides of the Tekezi river. ErDF are reportedly positioned in border areas of Eritrea and in SUDAN Kafta Humera Indasilassie % of incidents by type some kebeles in North-Western and Eastern zones. Thousands of people have been displaced from town Central Eastern these areas into Shire city, North-Western zone. In line with the Access Monitoring and Western Korarit https://bit.ly/3vcab7e May Reporting Framework: Electricity, telecommunications, and banking services continue to be disconnected throughout Tigray, Gaba Wukro Welkait TIGRAY 2% while commercial cargo and flights into the region remain suspended. This is having a major impact on Tselemti Abi Adi town May Tsebri relief operations. Partners are having to scale down operations and reduce movements due to the lack Dansha town town Mekelle AFAR 4% of fuel. -

Ethnobotany, Diverse Food Uses, Claimed Health Benefits And
Shewayrga and Sopade Journal of Ethnobiology and Ethnomedicine 2011, 7:19 http://www.ethnobiomed.com/content/7/1/19 JOURNAL OF ETHNOBIOLOGY AND ETHNOMEDICINE RESEARCH Open Access Ethnobotany, diverse food uses, claimed health benefits and implications on conservation of barley landraces in North Eastern Ethiopia highlands Hailemichael Shewayrga1* and Peter A Sopade2,3 Abstract Background: Barley is the number one food crop in the highland parts of North Eastern Ethiopia produced by subsistence farmers grown as landraces. Information on the ethnobotany, food utilization and maintenance of barley landraces is valuable to design and plan germplasm conservation strategies as well as to improve food utilization of barley. Methods: A study, involving field visits and household interviews, was conducted in three administrative zones. Eleven districts from the three zones, five kebeles in each district and five households from each kebele were visited to gather information on the ethnobotany, the utilization of barley and how barley end-uses influence the maintenance of landrace diversity. Results: According to farmers, barley is the “king of crops” and it is put for diverse uses with more than 20 types of barley dishes and beverages reportedly prepared in the study area. The products are prepared from either boiled/roasted whole grain, raw- and roasted-milled grain, or cracked grain as main, side, ceremonial, and recuperating dishes. The various barley traditional foods have perceived qualities and health benefits by the farmers. Fifteen diverse barley landraces were reported by farmers, and the ethnobotany of the landraces reflects key quantitative and qualitative traits. Some landraces that are preferred for their culinary qualities are being marginalized due to moisture shortage and soil degradation. -

Our Water Projects in 2018 Tigray Rural Water Supply and Hygiene
WellWishers Newsletter no. 46 March 2019 www.wellwishersethiopia.com Saving and enriching lives in Ethiopia Our water projects in 2018 Hand dug wells in Tigray: All successfully completed! REST have sent their final report for the 2018 program, and you can read it in full on the News page of our website. Wells were completed in 40 villages, providing a secure supply of clean water for 8621 people. Some of the important indicators in the final report show that, across the 40 wells: Water availability increased from 7.6 litres per day per person to 13.7 litres Average walking time to collect water reduced from 40 mins to 14 mins The percentage of girls going to school increased from 80% to 99%. Spring capping in Oromia: This was a new and exciting venture for WellWishers. Our new partner, Meki Branch Office of the Ethiopian Catholic Church Social & Development Commission proved to be up to the job and completed the spring capping for 1750 direct beneficiaries in Bombasso Reji Kebele, providing water for those within a 1.5- kilometer radius and at 15 litres per person per day. This project is 225 km south of Addis Ababa. Spring capping involves capturing clean water from a hillside spring, storing and making it available from pipes on the tank. The ongoing management of these two nearby systems will be by a locally trained committee of 3 women and 4 men. A final report from the partner is available on our website News page We are waiting on a new proposal from our partners in Meki, and it is likely that it will be to make this captured water available over a wider area through the provision of a reticulation system supported by solar-powered pumps. -

ETHIOPIA - National Hot Spot Map 31 May 2010
ETHIOPIA - National Hot Spot Map 31 May 2010 R Legend Eritrea E Tigray R egion !ª D 450 ho uses burned do wn d ue to th e re ce nt International Boundary !ª !ª Ahferom Sudan Tahtay Erob fire incid ent in Keft a hum era woreda. I nhabitan ts Laelay Ahferom !ª Regional Boundary > Mereb Leke " !ª S are repo rted to be lef t out o f sh elter; UNI CEF !ª Adiyabo Adiyabo Gulomekeda W W W 7 Dalul E !Ò Laelay togethe r w ith the regiona l g ove rnm ent is Zonal Boundary North Western A Kafta Humera Maychew Eastern !ª sup portin g the victim s with provision o f wate r Measle Cas es Woreda Boundary Central and oth er imm ediate n eeds Measles co ntinues to b e re ported > Western Berahle with new four cases in Arada Zone 2 Lakes WBN BN Tsel emt !A !ª A! Sub-city,Ad dis Ababa ; and one Addi Arekay> W b Afa r Region N b Afdera Military Operation BeyedaB Ab Ala ! case in Ahfe rom woreda, Tig ray > > bb The re a re d isplaced pe ople from fo ur A Debark > > b o N W b B N Abergele Erebtoi B N W Southern keb eles of Mille and also five kebeles B N Janam ora Moegale Bidu Dabat Wag HiomraW B of Da llol woreda s (400 0 persons) a ff ected Hot Spot Areas AWD C ases N N N > N > B B W Sahl a B W > B N W Raya A zebo due to flo oding from Awash rive r an d ru n Since t he beg in nin g of th e year, Wegera B N No Data/No Humanitarian Concern > Ziquala Sekota B a total of 967 cases of AWD w ith East bb BN > Teru > off fro m Tigray highlands, respective ly. -

Awareness of Community on Fishery and Aquaculture Production in Central Ethiopia
Alemu A. J Aquac Fisheries 2021, 5: 039 DOI: 10.24966/AAF-5523/100039 HSOA Journal of Aquaculture & Fisheries Research Article The domestic fishery of Africa involvement is projected to be Awareness of Community about 2.1 million tons of fish per year; it epitomizes 24% of the total world fish production from inland water bodies. The inland water on Fishery and Aquaculture body of Ethiopia is enclosed about 7,400 km2 of the lakes and about 7,000 km a total length of the rivers [2]. Further, 180 fish species were Production in Central Ethiopia harbored in these water bodies [3]. In Ethiopia, fish comes exclusively from inland water bodies with lakes, rivers, streams, reservoirs and substantial wetlands that are of great socio-economic, ecological and Tena Alemu * scientific importance [4,5]. Department of Animal Production and Technology, Wolkite University, Wol- kite, Ethiopia Ethiopia being a land locked country its fisheries is entirely based on inland water bodies, lakes, reservoirs and rivers. Fish production potential of the country is estimated to be 51,400 tonnes per annum [6]. Fishing has been the main source of protein supply for many Abstract people particularly for those who are residing in the locality of major water bodies like Lake Tana, Ziway, Awassa, Chamo, Baro River, etc The study was conducted in three different districts Gumer, [5]. Ethiopia is capable with numerous water bodies that cover a high Enemornaener and Cheha Woreda on awareness and perception of community on fishery and aquaculture production. In those diversity of aquatic wildlife. Reservoir fishery plays an important study areas majority of the people had the limitation of knowledge role in the economy of the country and the livelihoods of the people on production, consumption, and use of fish and aquaculture living adjacent to those reservoirs. -

The Impact of Fencing on Regeneration, Tree Growth and Carbon Stock in Desa Forest, Tigray, Ethiopia
ISSN: 2574-1241 Volume 5- Issue 4: 2018 DOI: 10.26717/BJSTR.2018.12.002183 Guo Ruo. Biomed J Sci & Tech Res Research Article Open Access The Impact of Fencing on Regeneration, Tree Growth and Carbon Stock in Desa Forest, Tigray, Ethiopia Guo Ruo1*, Brhane Weldegebrial2, Genet Yohannes3 and Gebremedhin Yohannes4 1College of Enviromental Science and Engineering, Tongjji University, China 2College of Environmental Science and Engineering, Tongjji University, China 3College of Agriculture and Natural Resources, Gambella University, Ethiopia 4College of Veterinary Medicine, Hawassa University, Ethiopia Received: : November 27, 2018; Published: : December 11, 2018 *Corresponding author: Guo Ruo, College of Enviromental Science and Engineering, Tongjji University, China Abstract Dryland forests of Ethiopia are facing a rapid rate of deforestation and degradation. This affects the growth and survival of plant density, diversity, growth and carbon stock. The aim of the study was to investigate the effect of fencing on regeneration, bonsai growth and carbon stock growth on permanent plots established in 2009. Data were analyzed using paired t-test to estimate any change on regeneration and carbon stock potential of Desa‟a forests. Data were collected on 24 plots of 20m×20m for trees and shrubs, 5m×5m for seedlings and on 40m×40m for bonsai carbon stock and bonsai growth between fenced and unfenced. Results showed that a total of 27 woody species were found representing 18 families. Oleawithin europaea the fenced subspecies and unfenced cuspidata block and over Juniperus time. However, procera were an independent with the highest t-test Importance was used to Value measure Index. any The significant overall diameter difference distribution on regeneration, showed an inverted J-shape indicating active regeneration status. -

Dairy Value Chain in West Amhara (Bahir Dar Zuria and Fogera Woreda Case)
Dairy Value Chain in West Amhara (Bahir Dar Zuria and Fogera Woreda case) Paulos Desalegn Commissioned by Programme for Agro-Business Induced Growth in the Amhara National Regional State August, 2018 Bahir Dar, Ethiopia 0 | Page List of Abbreviations and Acronyms AACCSA - Addis Ababa Chamber of Commerce and Sectorial Association AGP - Agriculture Growth Program AgroBIG – Agro-Business Induced Growth program AI - Artificial Insemination BZW - Bahir Dar Zuria Woreda CAADP - Comprehensive Africa Agriculture Development Program CIF - Cost, Insurance and Freight CSA - Central Statistics Agency ETB - Ethiopian Birr EU - European Union FAO - Food and Agriculture Organization of the United Nations FEED - Feed Enhancement for Ethiopian Development FGD - Focal Group Discussion FSP - Food Security Program FTC - Farmers Training Center GTP II - Second Growth and Transformation Plan KI - Key Informants KM (km) - Kilo Meter LIVES - Livestock and Irrigation Value chains for Ethiopian Smallholders LMD - Livestock Market Development LMP - Livestock Master Plan Ltr (ltr) - Liter PIF - Policy and Investment Framework USD - United States Dollar 1 | Page Table of Contents List of Abbreviations and Acronyms .................................................................................... 1 Executive summary ....................................................................................................... 3 List of Tables ............................................................................................................... 4 List of Figures -
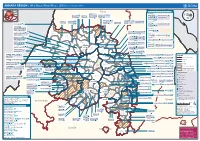
AMHARA REGION : Who Does What Where (3W) (As of 13 February 2013)
AMHARA REGION : Who Does What Where (3W) (as of 13 February 2013) Tigray Tigray Interventions/Projects at Woreda Level Afar Amhara ERCS: Lay Gayint: Beneshangul Gumu / Dire Dawa Plan Int.: Addis Ababa Hareri Save the fk Save the Save the df d/k/ CARE:f k Save the Children:f Gambela Save the Oromia Children: Children:f Children: Somali FHI: Welthungerhilfe: SNNPR j j Children:l lf/k / Oxfam GB:af ACF: ACF: Save the Save the af/k af/k Save the df Save the Save the Tach Gayint: Children:f Children: Children:fj Children:l Children: l FHI:l/k MSF Holand:f/ ! kj CARE: k Save the Children:f ! FHI:lf/k Oxfam GB: a Tselemt Save the Childrenf: j Addi Dessie Zuria: WVE: Arekay dlfk Tsegede ! Beyeda Concern:î l/ Mirab ! Concern:/ Welthungerhilfe:k Save the Children: Armacho f/k Debark Save the Children:fj Kelela: Welthungerhilfe: ! / Tach Abergele CRS: ak Save the Children:fj ! Armacho ! FHI: Save the l/k Save thef Dabat Janamora Legambo: Children:dfkj Children: ! Plan Int.:d/ j WVE: Concern: GOAL: Save the Children: dlfk Sahla k/ a / f ! ! Save the ! Lay Metema North Ziquala Children:fkj Armacho Wegera ACF: Save the Children: Tenta: ! k f Gonder ! Wag WVE: Plan Int.: / Concern: Save the dlfk Himra d k/ a WVE: ! Children: f Sekota GOAL: dlf Save the Children: Concern: Save the / ! Save: f/k Chilga ! a/ j East Children:f West ! Belesa FHI:l Save the Children:/ /k ! Gonder Belesa Dehana ! CRS: Welthungerhilfe:/ Dembia Zuria ! î Save thedf Gaz GOAL: Children: Quara ! / j CARE: WVE: Gibla ! l ! Save the Children: Welthungerhilfe: k d k/ Takusa dlfj k