Extended Endoscopic Endonasal Approach to the Pterygopalatine Fossa: Anatomical Study and Clinical Considerations
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Middle Cranial Fossa Sphenoidal Region Dural Arteriovenous Fistulas: Anatomic and Treatment Considerations
ORIGINAL RESEARCH INTERVENTIONAL Middle Cranial Fossa Sphenoidal Region Dural Arteriovenous Fistulas: Anatomic and Treatment Considerations Z.-S. Shi, J. Ziegler, L. Feng, N.R. Gonzalez, S. Tateshima, R. Jahan, N.A. Martin, F. Vin˜uela, and G.R. Duckwiler ABSTRACT BACKGROUND AND PURPOSE: DAVFs rarely involve the sphenoid wings and middle cranial fossa. We characterize the angiographic findings, treatment, and outcome of DAVFs within the sphenoid wings. MATERIALS AND METHODS: We reviewed the clinical and radiologic data of 11 patients with DAVFs within the sphenoid wing that were treated with an endovascular or with a combined endovascular and surgical approach. RESULTS: Nine patients presented with ocular symptoms and 1 patient had a temporal parenchymal hematoma. Angiograms showed that 5 DAVFs were located on the lesser wing of sphenoid bone, whereas the other 6 were on the greater wing of the sphenoid bone. Multiple branches of the ICA and ECA supplied the lesions in 7 patients. Four patients had cortical venous reflux and 7 patients had varices. Eight patients were treated with transarterial embolization using liquid embolic agents, while 3 patients were treated with transvenous embo- lization with coils or in combination with Onyx. Surgical disconnection of the cortical veins was performed in 2 patients with incompletely occluded DAVFs. Anatomic cure was achieved in all patients. Eight patients had angiographic and clinical follow-up and none had recurrence of their lesions. CONCLUSIONS: DAVFs may occur within the dura of the sphenoid wings and may often have a presentation similar to cavernous sinus DAVFs, but because of potential associations with the cerebral venous system, may pose a risk for intracranial hemorrhage. -

MR Imaging of the Orbital Apex
J Korean Radiol Soc 2000;4 :26 9-0 6 1 6 MR Imaging of the Orbital Apex: An a to m y and Pat h o l o g y 1 Ho Kyu Lee, M.D., Chang Jin Kim, M.D.2, Hyosook Ahn, M.D.3, Ji Hoon Shin, M.D., Choong Gon Choi, M.D., Dae Chul Suh, M.D. The apex of the orbit is basically formed by the optic canal, the superior orbital fis- su r e , and their contents. Space-occupying lesions in this area can result in clinical d- eficits caused by compression of the optic nerve or extraocular muscles. Even vas c u l a r changes in the cavernous sinus can produce a direct mass effect and affect the orbit ap e x. When pathologic changes in this region is suspected, contrast-enhanced MR imaging with fat saturation is very useful. According to the anatomic regions from which the lesions arise, they can be classi- fied as belonging to one of five groups; lesions of the optic nerve-sheath complex, of the conal and intraconal spaces, of the extraconal space and bony orbit, of the cav- ernous sinus or diffuse. The characteristic MR findings of various orbital lesions will be described in this paper. Index words : Orbit, diseases Orbit, MR The apex of the orbit is a complex region which con- tains many nerves, vessels, soft tissues, and bony struc- Anatomy of the orbital apex tures such as the superior orbital fissure and the optic canal (1-3), and is likely to be involved in various dis- The orbital apex region consists of the optic nerve- eases (3). -

Palatal Injection Does Not Block the Superior Alveolar Nerve Trunks: Correcting an Error Regarding the Innervation of the Maxillary Teeth
Open Access Review Article DOI: 10.7759/cureus.2120 Palatal Injection does not Block the Superior Alveolar Nerve Trunks: Correcting an Error Regarding the Innervation of the Maxillary Teeth Joe Iwanaga 1 , R. Shane Tubbs 2 1. Seattle Science Foundation 2. Neurosurgery, Seattle Science Foundation Corresponding author: Joe Iwanaga, [email protected] Abstract The superior alveolar nerves course lateral to the maxillary sinus and the greater palatine nerve travels through the hard palate. This difficult three-dimensional anatomy has led some dentists and oral surgeons to a critical misunderstanding in developing the anterior and middle superior alveolar (AMSA) nerve block and the palatal approach anterior superior alveolar (P-ASA) nerve block. In this review, the anatomy of the posterior, middle and anterior superior alveolar nerves, greater palatine nerve, and nasopalatine nerve are revisited in order to clarify the anatomy of these blocks so that the perpetuated anatomical misunderstanding is rectified. We conclude that the AMSA and P-ASA nerve blockades, as currently described, are not based on accurate anatomy. Categories: Anesthesiology, Medical Education, Other Keywords: anatomy, innervation, local anesthesia, maxillary nerve, nerve block, tooth Introduction And Background Anesthetic blockade of the posterior superior alveolar (PSA) branch of the maxillary nerve has played an important role in the endodontic treatment of irreversible acute pulpitis of the upper molar teeth except for the mesiobuccal root of the first molar tooth [1, 2]. This procedure requires precise anatomical knowledge of the pterygopalatine fossa and related structures in order to avoid unnecessary complications and to make the blockade most effective. The infraorbital nerve gives rise to middle superior alveolar (MSA) and anterior superior alveolar (ASA) branches. -

Craniotomy for Anterior Cranial Fossa Meningiomas: Historical Overview
Neurosurg Focus 36 (4):E14, 2014 ©AANS, 2014 Craniotomy for anterior cranial fossa meningiomas: historical overview SAUL F. MORALES-VALERO, M.D., JAMIE J. VAN GOMPEL, M.D., IOANNIS LOUMIOTIS, M.D., AND GIUSEPPE LANZINO, M.D. Department of Neurologic Surgery, Mayo Clinic, Mayo Medical School, Rochester, Minnesota The surgical treatment of meningiomas located at the base of the anterior cranial fossa is often challenging, and the evolution of the surgical strategy to resect these tumors parallels the development of craniotomy, and neurosur- gery in general, over the past century. Early successful operations to treat these tumors were pioneered by prominent figures such as Sir William Macewen and Francesco Durante. Following these early reports, Harvey Cushing made significant contributions, allowing a better understanding and treatment of meningiomas in general, but particularly those involving the anterior cranial base. Initially, large-sized unilateral or bilateral craniotomies were necessary to approach these deep-seated lesions. Technical advances such as the introduction of electrosurgery, the operating microscope, and refined microsurgical instruments allowed neurosurgeons to perform less invasive surgical proce- dures with better results. Today, a wide variety of surgical strategies, including endoscopic surgery and radiosurgery, are used to treat these tumors. In this review, the authors trace the evolution of craniotomy for anterior cranial fossa meningiomas. (http://thejns.org/doi/abs/10.3171/2014.1.FOCUS13569) KEY WORDS • intracranial meningiomas • craniotomy • history • anterior cranial fossa ENINGIOMAS of the anterior cranial fossa represent has a few distinct clinical features. However, in practice, 12%–20% of all intracranial meningiomas.5,30 this group of tumors often represents a continuum. -

The Anatomic Analysis of the Vidian Canal and the Surrounding
Braz J Otorhinolaryngol. 2019;85(2):136---143 Brazilian Journal of OTORHINOLARYNGOLOGY www.bjorl.org ORIGINAL ARTICLE The anatomic analysis of the vidian canal and the surrounding structures concerning vidian neurectomy ଝ using computed tomography scans a,∗ a b Gülay Ac¸ar , Aynur Emine C¸ic¸ekcibas¸ı , ˙Ibrahim C¸ukurova , c a d Kemal Emre Özen , Muzaffer ¸ekerS , ˙Ibrahim Güler a Necmettin Erbakan University, Meram Faculty of Medicine, Department of Anatomy, Konya, Turkey b Health Sciences University, Izmir Tepecik Trainig and Research Hospital, Department of Otolaryngology-Head and Neck Surgery, Izmir, Turkey c Katip C¸elebi University, Faculty of Medicine, Department of Anatomy, Izmir, Turkey d Selcuk University, Faculty of Medicine, Department of Radiology, Konya, Turkey Received 15 September 2017; accepted 8 November 2017 Available online 26 December 2017 KEYWORDS Abstract Intrasphenoid Introduction: The type of endoscopic approach chosen for vidian neurectomy can be specified septum; by evaluating the vidian canal and the surrounding sphenoid sinus structures. Morphometric Objective: The variations and morphometry of the vidian canal were investigated, focusing on analysis; the functional correlations between them which are crucial anatomical landmarks for preoper- Pterygoid process ative planning. pneumatization; Methods: This study was performed using paranasal multidetector computed tomography Vidian canal; images that were obtained with a section thickening of 0.625 mm of 250 adults. Vidian neurectomy Results: The distributions of 500 vidian canal variants were categorized as follows; Type 1, within the sphenoid corpus (55.6%); Type 2, partially protruding into the sphenoid sinus (34.8%); Type 3, within the sphenoid sinus (9.6%). The pneumatization of the pterygoid process is mostly seen in vidian canal Type 2 (72.4%) and Type 3 (95.8%) (p < 0.001). -

Anatomy of Maxillary and Mandibular Local Anesthesia
Anatomy of Mandibular and Maxillary Local Anesthesia Patricia L. Blanton, Ph.D., D.D.S. Professor Emeritus, Department of Anatomy, Baylor College of Dentistry – TAMUS and Private Practice in Periodontics Dallas, Texas Anatomy of Mandibular and Maxillary Local Anesthesia I. Introduction A. The anatomical basis of local anesthesia 1. Infiltration anesthesia 2. Block or trunk anesthesia II. Review of the Trigeminal Nerve (Cranial n. V) – the major sensory nerve of the head A. Ophthalmic Division 1. Course a. Superior orbital fissure – root of orbit – supraorbital foramen 2. Branches – sensory B. Maxillary Division 1. Course a. Foramen rotundum – pterygopalatine fossa – inferior orbital fissure – floor of orbit – infraorbital 2. Branches - sensory a. Zygomatic nerve b. Pterygopalatine nerves [nasal (nasopalatine), orbital, palatal (greater and lesser palatine), pharyngeal] c. Posterior superior alveolar nerves d. Infraorbital nerve (middle superior alveolar nerve, anterior superior nerve) C. Mandibular Division 1. Course a. Foramen ovale – infratemporal fossa – mandibular foramen, Canal -> mental foramen 2. Branches a. Sensory (1) Long buccal nerve (2) Lingual nerve (3) Inferior alveolar nerve -> mental nerve (4) Auriculotemporal nerve b. Motor (1) Pterygoid nerves (2) Temporal nerves (3) Masseteric nerves (4) Nerve to tensor tympani (5) Nerve to tensor veli palatine (6) Nerve to mylohyoid (7) Nerve to anterior belly of digastric c. Both motor and sensory (1) Mylohyoid nerve III. Usual Routes of innervation A. Maxilla 1. Teeth a. Molars – Posterior superior alveolar nerve b. Premolars – Middle superior alveolar nerve c. Incisors and cuspids – Anterior superior alveolar nerve 2. Gingiva a. Facial/buccal – Superior alveolar nerves b. Palatal – Anterior – Nasopalatine nerve; Posterior – Greater palatine nerves B. -
![View (FOV) 210, Number of in a Level C Recommendation for INALA for Acute Acquisitions 3; Sagittal T1 Weighted: TR Range 710, TE 10, Migraine Treatment [6]](https://docslib.b-cdn.net/cover/6766/view-fov-210-number-of-in-a-level-c-recommendation-for-inala-for-acute-acquisitions-3-sagittal-t1-weighted-tr-range-710-te-10-migraine-treatment-6-496766.webp)
View (FOV) 210, Number of in a Level C Recommendation for INALA for Acute Acquisitions 3; Sagittal T1 Weighted: TR Range 710, TE 10, Migraine Treatment [6]
Crespi et al. The Journal of Headache and Pain (2018) 19:14 The Journal of Headache https://doi.org/10.1186/s10194-018-0843-5 and Pain RESEARCHARTICLE Open Access Measurement and implications of the distance between the sphenopalatine ganglion and nasal mucosa: a neuroimaging study Joan Crespi1,2,3* , Daniel Bratbak2,4, David Dodick2,5, Manjit Matharu6, Kent Are Jamtøy2,7, Irina Aschehoug2 and Erling Tronvik1,2,3 Abstract Background: Historical reports describe the sphenopalatine ganglion (SPG) as positioned directly under the nasal mucosa. This is the basis for the topical intranasal administration of local anaesthetic (LA) towards the sphenopalatine foramen (SPF) which is hypothesized to diffuse a distance as short as 1 mm. Nonetheless, the SPG is located in the sphenopalatine fossa, encapsulated in connective tissue, surrounded by fat tissue and separated from the nasal cavity by a bony wall. The sphenopalatine fossa communicates with the nasal cavity through the SPF, which contains neurovascular structures packed with connective tissue and is covered by mucosa in the nasal cavity. Endoscopically the SPF does not appear open. It has hitherto not been demonstrated that LA reaches the SPG using this approach. Methods: Our group has previously identified the SPG on 3 T–MRI images merged with CT. This enabled us to measure the distance from the SPG to the nasal mucosa covering the SPF in 20 Caucasian subjects on both sides (n =40ganglia). This distance was measured by two physicians. Interobserver variability was evaluated using the intraclass correlation coefficient (ICC). Results: The mean distance from the SPG to the closest point of the nasal cavity directly over the mucosa covering the SPF was 6.77 mm (SD 1.75; range, 4.00–11.60). -

Morfofunctional Structure of the Skull
N.L. Svintsytska V.H. Hryn Morfofunctional structure of the skull Study guide Poltava 2016 Ministry of Public Health of Ukraine Public Institution «Central Methodological Office for Higher Medical Education of MPH of Ukraine» Higher State Educational Establishment of Ukraine «Ukranian Medical Stomatological Academy» N.L. Svintsytska, V.H. Hryn Morfofunctional structure of the skull Study guide Poltava 2016 2 LBC 28.706 UDC 611.714/716 S 24 «Recommended by the Ministry of Health of Ukraine as textbook for English- speaking students of higher educational institutions of the MPH of Ukraine» (minutes of the meeting of the Commission for the organization of training and methodical literature for the persons enrolled in higher medical (pharmaceutical) educational establishments of postgraduate education MPH of Ukraine, from 02.06.2016 №2). Letter of the MPH of Ukraine of 11.07.2016 № 08.01-30/17321 Composed by: N.L. Svintsytska, Associate Professor at the Department of Human Anatomy of Higher State Educational Establishment of Ukraine «Ukrainian Medical Stomatological Academy», PhD in Medicine, Associate Professor V.H. Hryn, Associate Professor at the Department of Human Anatomy of Higher State Educational Establishment of Ukraine «Ukrainian Medical Stomatological Academy», PhD in Medicine, Associate Professor This textbook is intended for undergraduate, postgraduate students and continuing education of health care professionals in a variety of clinical disciplines (medicine, pediatrics, dentistry) as it includes the basic concepts of human anatomy of the skull in adults and newborns. Rewiewed by: O.M. Slobodian, Head of the Department of Anatomy, Topographic Anatomy and Operative Surgery of Higher State Educational Establishment of Ukraine «Bukovinian State Medical University», Doctor of Medical Sciences, Professor M.V. -

Clinical Importance of the Middle Meningeal Artery
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Jagiellonian Univeristy Repository FOLIA MEDICA CRACOVIENSIA 41 Vol. LIII, 1, 2013: 41–46 PL ISSN 0015-5616 Przemysław Chmielewski1, Janusz skrzat1, Jerzy waloCha1 CLINICAL IMPORTANCE OF THE MIDDLE MENINGEAL ARTERY Abstract: Middle meningeal artery (MMA)is an important branch which supplies among others cranial dura mater. It directly attaches to the cranial bones (is incorporated into periosteal layer of dura mater), favors common injuries in course of head trauma. This review describes available data on the MMA considering its varability, or treats specific diseases or injuries where the course of MMA may have clinical impact. Key words: Middle meningeal artery (MMA), aneurysm of the middle meningeal artery, epidural he- matoma, anatomical variation of MMA. TOPOGRAPHY OF THE MIDDLE MENINGEAL ARTERY AND ITS BRANCHES Middle meningeal artery (MMA) [1] is most commonly the strongest branch of maxillary artery (from external carotid artery) [2]. It supplies blood to cranial dura mater, and through the numerous perforating branches it nourishes also periosteum of the inner aspect of cranial bones. It enters the middle cranial fossa through the foramen spinosum, and courses between the dura mater and the inner aspect of the vault of the skull. Next it divides into two terminal branches — frontal (anterior) which supplies blood to bones forming anterior cranial fossa and the anterior part of the middle cranial fossa; parietal branch (posterior), which runs more horizontally toward the back and supplies posterior part of the middle cranial fossa and supratentorial part of the posterior cranial fossa. -
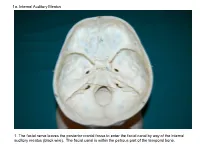
1A. Internal Auditory Meatus
1a. Internal Auditory Meatus 1. The facial nerve leaves the posterior cranial fossa to enter the facial canal by way of the internal auditory meatus (black wire). The facial canal is within the petrous part of the temporal bone. 1b. Internal Auditory Meatus The facial nerve leaves the posterior cranial fossa to enter the facial canal by way of the internal auditory meatus (black wire). 2. Hiatus of the Canal and Groove for the Greater Superficial Petrosal Nerve The greater superficial petrosal nerve leaves the facial canal to enter the middle cranial fossa by way of the hiatus of the canal for the greater superficial petrosal nerve (black wire). 3. Pterygoid Canal at Anterior Lip of the Lacerate Foramen The greater superficial petrosal nerve is joined by the deep petrosal nerve to form the nerve of the pterygoid canal (black and red wire). This nerve leaves the middle cranial fossa to enter the pterygopalatine fossa by way of the pterygoid canal. The posterior opening of the pterygoid canal is at the anterior lip of the lacerate foramen. The greater superficial nerve and the deep petrosal nerve travel within the cavernous sinus. 4. Pterygopalatine Fossa Seen Through the Pterygomaxillary Fissure The anterior opening of the pterygoid canal is into the pterygopalatine fossa (black wire). The pterygopalatine fossa is located medial to the pterygomaxillary fissure and contains the pterygopalatine ganglion. 5. External Auditory Meatus The chorda tympani nerve leaves the facial canal and crosses the middle ear (black wire). It then leaves the middle ear to arrive in the infratemporal fossa by way of the petrotympanic fissure. -

Spontaneous Encephaloceles of the Temporal Lobe
Neurosurg Focus 25 (6):E11, 2008 Spontaneous encephaloceles of the temporal lobe JOSHUA J. WIND , M.D., ANTHONY J. CAPUTY , M.D., AND FABIO ROBE R TI , M.D. Department of Neurological Surgery, George Washington University, Washington, DC Encephaloceles are pathological herniations of brain parenchyma through congenital or acquired osseus-dural defects of the skull base or cranial vault. Although encephaloceles are known as rare conditions, several surgical re- ports and clinical series focusing on spontaneous encephaloceles of the temporal lobe may be found in the otological, maxillofacial, radiological, and neurosurgical literature. A variety of symptoms such as occult or symptomatic CSF fistulas, recurrent meningitis, middle ear effusions or infections, conductive hearing loss, and medically intractable epilepsy have been described in patients harboring spontaneous encephaloceles of middle cranial fossa origin. Both open procedures and endoscopic techniques have been advocated for the treatment of such conditions. The authors discuss the pathogenesis, diagnostic assessment, and therapeutic management of spontaneous temporal lobe encepha- loceles. Although diagnosis and treatment may differ on a case-by-case basis, review of the available literature sug- gests that spontaneous encephaloceles of middle cranial fossa origin are a more common pathology than previously believed. In particular, spontaneous cases of posteroinferior encephaloceles involving the tegmen tympani and the middle ear have been very well described in the medical literature. -

Functional Structure of the Skull and Fractures of the Skull Thickened and Thinner Parts of the Skull
Functional structure of the skull and Fractures of the skull Thickened and thinner parts of the skull = important base for understanding of the functional structure of the skull → - the transmission of masticatory forces - fracture predilection Thickned parts: . sagittal line . ventral lateral line . dorsal lateral line Thinner parts: . articular fossa . cribriform plate . foramines, canals and fissures . anterior, medial and posterior cranial fossa Thickned parts: . tuber parietalis . mastoid process . protuberantia occipitalis ext. et int. linea temporalis . margin of sulcus sinus: - sagitalis sup. - transversus Functional structure of the skull Facial buttresses system . Of thin segments of bone encased and supported by a more rigid framework of "buttresses" . The midface is anchored to the cranium through this framework . Is formed by strong frontal, maxillary, zygomatic and sphenoid bones and their attachments to one another Tuber maxillae Vertical buttress Sinus maxillae Orbita . nasomaxillary Nasal cavity . zygomaticomaxillary . pterygomaxillary Horizontal buttress . glabella . orbital rims . zygomatic processes . maxillary palate . The buttress system absorbs and transmits forces applied to the facial skeleton . Masticatory forces are transmitted to the skull base primarily through the vertical buttresses, which are joined and additionally supported by the horizontal buttresses . When external forces are applied, these components prevent disruption of the facial skeleton until a critical level is reached and then fractures occur Stress that occurs from mastication or trauma is transferred from the inferior of the mandible via various trajectory lines → to the condyles glenoid fossa → temporal bone The main alveolar stress concentration were located interradicularly and interproximally Fractures of the skull I. Neurocranial fractures II. Craniofacial fractures I. Neurocranial fracture . A break in the skull bone are generally occurs as a result of a direct impact .