165191 Chap.6.6F
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Ocean Deoxygenation and Copepods: Coping with Oxygen Minimum Zone Variability
Biogeosciences, 17, 2315–2339, 2020 https://doi.org/10.5194/bg-17-2315-2020 © Author(s) 2020. This work is distributed under the Creative Commons Attribution 4.0 License. Ocean deoxygenation and copepods: coping with oxygen minimum zone variability Karen F. Wishner1, Brad Seibel2, and Dawn Outram1 1Graduate School of Oceanography, University of Rhode Island, Narragansett, RI 02882, USA 2College of Marine Science, University of South Florida, St. Petersburg, FL 33701, USA Correspondence: Karen F. Wishner ([email protected]) Received: 27 September 2019 – Discussion started: 28 October 2019 Revised: 31 March 2020 – Accepted: 2 April 2020 – Published: 24 April 2020 Abstract. Increasing deoxygenation (loss of oxygen) of compression concept). These distribution depths changed by the ocean, including expansion of oxygen minimum zones tens to hundreds of meters depending on the species, oxygen (OMZs), is a potentially important consequence of global profile, and phenomenon. For example, at the lower oxycline, warming. We examined present-day variability of vertical the depth of maximum abundance for Lucicutia hulsemannae distributions of 23 calanoid copepod species in the East- shifted from ∼ 600 to ∼ 800 m, and the depth of diapause for ern Tropical North Pacific (ETNP) living in locations with Eucalanus inermis shifted from ∼ 500 to ∼ 775 m, in an ex- different water column oxygen profiles and OMZ inten- panded OMZ compared to a thinner OMZ, but remained at sity (lowest oxygen concentration and its vertical extent in similar low oxygen levels in -

Tesis Estructura Comunitaria De Copepodos .Pdf
Universidad de Concepción Dirección de Postgrado Facultad de Ciencias Naturales y Oceanográficas Programa de Magister en Ciencias mención Oceanografía Estructura comunitaria de copépodos pelágicos asociados a montes submarinos de la Dorsal Juan Fernández (32-34°S) en el Pacífico Sur Oriental Tesis para optar al grado de Magíster en Ciencias con mención en Oceanografía PAMELA ANDREA FIERRO GONZÁLEZ CONCEPCIÓN-CHILE 2019 Profesora Guía: Pamela Hidalgo Díaz Departamento de Oceanografía, Facultad de Ciencias Naturales y Oceanográficas Universidad de Concepción Profesor Co-guía: Rubén Escribano Departamento de Oceanografía, Facultad de Ciencias Naturales y Oceanográficas Universidad de Concepción La Tesis de “Magister en Ciencias con mención en Oceanografía” titulada “Estructura comunitaria de copépodos pelágicos asociados a montes submarinos de la Dorsal Juan Fernández (32-34°S) en el Pacífico sur oriental”, de la Srta. “PAMELA ANDREA FIERRO GONZÁLEZ” y realizada bajo la Facultad de Ciencias Naturales y Oceanográficas, Universidad de Concepción, ha sido aprobada por la siguiente Comisión de Evaluación: Dra. Pamela Hidalgo Díaz Profesora Guía Universidad de Concepción Dr. Rubén Escribano Profesor Co-Guía Universidad de Concepción Dr. Samuel Hormazábal Miembro de la Comisión Evaluadora Pontificia Universidad Católica de Valparaíso Dr. Fabián Tapia Director Programa de Magister en Oceanografía Universidad de Concepción ii A Juan Carlos y Sebastián iii AGRADECIMIENTOS Agradezco a quienes con su colaboración y apoyo hicieron posible el desarrollo y término de esta tesis. En primer lugar, agradezco a los miembros de mi comisión de tesis. A mi profesora guía, Dra. Pamela Hidalgo, por apoyarme y guiarme en este largo camino de formación académica, por su gran calidad humana, contención y apoyo personal. -

Molecular Species Delimitation and Biogeography of Canadian Marine Planktonic Crustaceans
Molecular Species Delimitation and Biogeography of Canadian Marine Planktonic Crustaceans by Robert George Young A Thesis presented to The University of Guelph In partial fulfilment of requirements for the degree of Doctor of Philosophy in Integrative Biology Guelph, Ontario, Canada © Robert George Young, March, 2016 ABSTRACT MOLECULAR SPECIES DELIMITATION AND BIOGEOGRAPHY OF CANADIAN MARINE PLANKTONIC CRUSTACEANS Robert George Young Advisors: University of Guelph, 2016 Dr. Sarah Adamowicz Dr. Cathryn Abbott Zooplankton are a major component of the marine environment in both diversity and biomass and are a crucial source of nutrients for organisms at higher trophic levels. Unfortunately, marine zooplankton biodiversity is not well known because of difficult morphological identifications and lack of taxonomic experts for many groups. In addition, the large taxonomic diversity present in plankton and low sampling coverage pose challenges in obtaining a better understanding of true zooplankton diversity. Molecular identification tools, like DNA barcoding, have been successfully used to identify marine planktonic specimens to a species. However, the behaviour of methods for specimen identification and species delimitation remain untested for taxonomically diverse and widely-distributed marine zooplanktonic groups. Using Canadian marine planktonic crustacean collections, I generated a multi-gene data set including COI-5P and 18S-V4 molecular markers of morphologically-identified Copepoda and Thecostraca (Multicrustacea: Hexanauplia) species. I used this data set to assess generalities in the genetic divergence patterns and to determine if a barcode gap exists separating interspecific and intraspecific molecular divergences, which can reliably delimit specimens into species. I then used this information to evaluate the North Pacific, Arctic, and North Atlantic biogeography of marine Calanoida (Hexanauplia: Copepoda) plankton. -

Observing Copepods Through a Genomic Lens James E Bron1*, Dagmar Frisch2, Erica Goetze3, Stewart C Johnson4, Carol Eunmi Lee5 and Grace a Wyngaard6
Bron et al. Frontiers in Zoology 2011, 8:22 http://www.frontiersinzoology.com/content/8/1/22 DEBATE Open Access Observing copepods through a genomic lens James E Bron1*, Dagmar Frisch2, Erica Goetze3, Stewart C Johnson4, Carol Eunmi Lee5 and Grace A Wyngaard6 Abstract Background: Copepods outnumber every other multicellular animal group. They are critical components of the world’s freshwater and marine ecosystems, sensitive indicators of local and global climate change, key ecosystem service providers, parasites and predators of economically important aquatic animals and potential vectors of waterborne disease. Copepods sustain the world fisheries that nourish and support human populations. Although genomic tools have transformed many areas of biological and biomedical research, their power to elucidate aspects of the biology, behavior and ecology of copepods has only recently begun to be exploited. Discussion: The extraordinary biological and ecological diversity of the subclass Copepoda provides both unique advantages for addressing key problems in aquatic systems and formidable challenges for developing a focused genomics strategy. This article provides an overview of genomic studies of copepods and discusses strategies for using genomics tools to address key questions at levels extending from individuals to ecosystems. Genomics can, for instance, help to decipher patterns of genome evolution such as those that occur during transitions from free living to symbiotic and parasitic lifestyles and can assist in the identification of genetic mechanisms and accompanying physiological changes associated with adaptation to new or physiologically challenging environments. The adaptive significance of the diversity in genome size and unique mechanisms of genome reorganization during development could similarly be explored. -
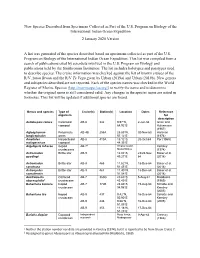
New Species Described from Specimens Collected As Part of the U.S
New Species Described from Specimens Collected as Part of the U.S. Program on Biology of the International Indian Ocean Expedition 2 January 2020 Version A list was generated of the species described based on specimens collected as part of the U.S. Program on Biology of the International Indian Ocean Expedition. This list was compiled from a search of publications cited by scientists involved in the U.S. Program on Biology and publications held by the Smithsonian Institution. The list includes holotypes and paratypes used to describe species. The cruise information was checked against the list of known cruises of the R/V Anton Bruun and the R/V Te Vega given by Urban (2019a) and Urban (2019b). New genera and subspecies described are not reported. Each of the species names was checked in the World Register of Marine Species (http://marinespecies.org/) to verify the name and to determine whether the original name is still considered valid. Any changes in the species name are noted in footnotes. This list will be updated if additional species are found. Genus and species Type of Cruise(s) Station(s) Location Dates Reference organism for description Aetideopsis retusa Calanaoid AB-6 342 9.97°S, 2-Jun-64 Grice and copepod 64.92°E Hulsemann (1967) Aglaophamus Polychaete AB-4B 255A 25.83°N, 30-Nov-63 Hartman longicephalus worm 57.12°E (1974) Ameliotes Harpacticoid AB-8 410A 15.12°S, 20-Oct-64 Por (1969) malagassicus copepod 44.35°E Argeiopsis inhacae isopod AB-71 Inhaca Island, Kensley crustaceans Mozambique (1974) Astrocladus Brittle star AB-9 12.83°S, 23/26-Nov- Baker et al. -

Four New Species of Heteromysis (Crustacea: Mysida) from Public Aquaria in Hawaii, Florida, and Western to Central Europe
European Journal of Taxonomy 735: 133–175 ISSN 2118-9773 https://doi.org/10.5852/ejt.2021.735.1247 www.europeanjournaloftaxonomy.eu 2021 · Wittmann K.J. & Abed-Navandi D. This work is licensed under a Creative Commons Attribution License (CC BY 4.0). Research article urn:lsid:zoobank.org:pub:F1CE3697-319D-4D02-A99F-11A0E16A8743 Four new species of Heteromysis (Crustacea: Mysida) from public aquaria in Hawaii, Florida, and Western to Central Europe Karl J. WITTMANN 1,* & Daniel ABED-NAVANDI 2 1 Medical University of Vienna, Department of Environmental Health, Kinderspitalgasse 15, 1090 Vienna, Austria. 2 Haus des Meeres – Aqua Terra Zoo, Fritz Grünbaum Platz 1, 1060 Vienna, Austria. * Corresponding author: [email protected] 2 Email: [email protected] 1 urn:lsid:zoobank.org:author:C90E7BC4-A27A-4B41-93F3-6224D17795FF 2 urn:lsid:zoobank.org:author:179B83B0-8C8B-4DF5-8986-4227B8E1BB9B Abstract. Four new species of the subgenus Heteromysis (Olivemysis) were detected in material from (sub)-tropical aquaria in six public aquarium institutions around the globe. Modifications of pleopods by spines represent the strongest structural complex used for differentiation within this subgenus: male pleopods 1–4 modified in H. smithsoniana sp. nov., male pleopods 2–4 plus female pleopod 2 in H. hornimani sp. nov. and H. waikikensis sp. nov. Additional important diagnostic characters are provided by the antennulae, uropods, and telson. The male of H. sixi sp. nov. represents a very rare case within the genus Heteromysis by having only pleopod 2 modified by flagellate spines. The definition of the subgenus Olivemysis is modified in order to include H. -

Revising the Definition of the Crustacean Seta and Setal
Blackwell Science, LtdOxford, UKZOJZoological Journal of the Linnean Society0024-4082The Lin- nean Society of London, 2004? 2004 142? 233252 Original Article DEFINITION OF CRUSTACEAN SETAE AND SETAL CLASSIFICATION SYSTEMA. GARM Zoological Journal of the Linnean Society, 2004, 142, 233–252. With 11 figures Revising the definition of the crustacean seta and setal classification systems based on examinations of the mouthpart setae of seven species of decapods A. GARM* Biological Institute, University of Copenhagen, Denmark Received July 2003; accepted for publication June 2004 The crustacean cuticle has numerous projections and some of these projections, the setae, have important mechan- ical as well as sensory functions. The setae display a wide diversity in their external morphology, which has led to great problems separating setae from other projections in the cuticle and problems in making a consistent classifi- cation system. Here, the cuticular projections on the mouthparts of seven species of decapods are examined by scan- ning and transmission electron microscopy. A new definition is given: a seta is an elongate projection with a more or less circular base and a continuous lumen; the lumen has a semicircular arrangement of sheath cells basally. From the details of the external morphology the mouthpart setae are divided into seven types: pappose, plumose, serrulate, serrate, papposerrate, simple and cuspidate setae, which are suggested to reflect mechanical functions and not evolutionary history. This classification system is compared with earlier systems. © 2004 The Linnean Society of London, Zoological Journal of the Linnean Society, 2004, 142, 233–252. ADDITIONAL KEYWORDS: functional morphology – mechanical function – setal types. INTRODUCTION paper, the most often used term is setae and it will therefore be used here. -

Vertical Distribution and Seasonal Variation of Pelagic Chaetognaths in Sagami Bay, Central Japan
Plankton Benthos Res 7(2): 41–54, 2012 Plankton & Benthos Research © The Plankton Society of Japan Vertical distribution and seasonal variation of pelagic chaetognaths in Sagami Bay, central Japan 1,2, 2 2 1 HIROOMI MIYAMOTO *, SHUHEI NISHIDA , KAZUNORI KURODA & YUJI TANAKA 1 Graduate School of Marine Science and Technology, Tokyo University of Marine Science and Technology, 4–5–7 Konan, Minato-ku, Tokyo 108–8477, Japan 2 Atmosphere and Ocean Research Institute, University of Tokyo, 5–1–5 Kashiwanoha, Kashiwa, Chiba 277–8564, Japan Received 22 November 2011; Accepted 24 February 2012 Abstract: The vertical distribution and seasonal variation of pelagic chaetognaths was investigated in Sagami Bay, based on stratified zooplankton samples from the upper 1,400 m. The chaetognaths were most abundant in the 100– 150 m layer in January and May 2005, whereas they were concentrated in the upper 50 m in the other months. Among the 28 species identified, Zonosagitta nagae had the highest mean standing stock, followed by Flaccisagitta enflata and Eukrohnia hamata. Cluster analysis based on species composition and density separated chaetognath communi- ties into four groups (Groups A–D). While the distribution of Group C was unclear due to their rare occurrence, the other groups were more closely associated with depth than with season. The epipelagic group (Group A) was further divided into four sub-groups, which were related to seasonal hydrographic variation. The mesopelagic group (Group B) was mainly composed of samples from the 150–400 m layer, although Group A, in which the epipelagic species Z. nagae dominated, was distributed in this layer from May to July. -

Clausocalanus Giesbrecht, 1888
Clausocalanus Giesbrecht, 1888 Maria Grazia Mazzocchi Leaflet No. 189 I April 2020 ICES IDENTIFICATION LEAFLETS FOR PLANKTON FICHES D’IDENTIFICATION DU ZOOPLANCTON ICES INTERNATIONAL COUNCIL FOR THE EXPLORATION OF THE SEA CIEM CONSEIL INTERNATIONAL POUR L’EXPLORATION DE LA MER International Council for the Exploration of the Sea Conseil International pour l’Exploration de la Mer H. C. Andersens Boulevard 44–46 DK-1553 Copenhagen V Denmark Telephone (+45) 33 38 67 00 Telefax (+45) 33 93 42 15 www.ices.dk [email protected] Series editor: Antonina dos Santos and Lidia Yebra Prepared under the auspices of the ICES Working Group on Zooplankton Ecology (WGZE) This leaflet has undergone a formal external peer-review process Recommended format for purpose of citation: Mazzocchi, M.G. 2020. Clausocalanus Giesbrecht, 1888. ICES Identification Leaflets for Plankton No. 189. 19 pp. http://doi.org/10.17895/ices.pub.5464 The material in this report may be reused for non-commercial purposes using the recommended citation. ICES may only grant usage rights of information, data, images, graphs, etc. of which it has ownership. For other third-party material cited in this report, you must contact the original copyright holder for permis-sion. For citation of datasets or use of data to be included in other databases, please refer to the latest ICES data policy on the ICES website. All extracts must be acknowledged. For other reproduction requests please contact the General Secretary. This document is the product of an expert group under the auspices of the International Council for the Exploration of the Sea and does not necessarily represent the view of the Council. -

Southeastern Regional Taxonomic Center South Carolina Department of Natural Resources
Southeastern Regional Taxonomic Center South Carolina Department of Natural Resources http://www.dnr.sc.gov/marine/sertc/ Southeastern Regional Taxonomic Center Invertebrate Literature Library (updated 9 May 2012, 4056 entries) (1958-1959). Proceedings of the salt marsh conference held at the Marine Institute of the University of Georgia, Apollo Island, Georgia March 25-28, 1958. Salt Marsh Conference, The Marine Institute, University of Georgia, Sapelo Island, Georgia, Marine Institute of the University of Georgia. (1975). Phylum Arthropoda: Crustacea, Amphipoda: Caprellidea. Light's Manual: Intertidal Invertebrates of the Central California Coast. R. I. Smith and J. T. Carlton, University of California Press. (1975). Phylum Arthropoda: Crustacea, Amphipoda: Gammaridea. Light's Manual: Intertidal Invertebrates of the Central California Coast. R. I. Smith and J. T. Carlton, University of California Press. (1981). Stomatopods. FAO species identification sheets for fishery purposes. Eastern Central Atlantic; fishing areas 34,47 (in part).Canada Funds-in Trust. Ottawa, Department of Fisheries and Oceans Canada, by arrangement with the Food and Agriculture Organization of the United Nations, vols. 1-7. W. Fischer, G. Bianchi and W. B. Scott. (1984). Taxonomic guide to the polychaetes of the northern Gulf of Mexico. Volume II. Final report to the Minerals Management Service. J. M. Uebelacker and P. G. Johnson. Mobile, AL, Barry A. Vittor & Associates, Inc. (1984). Taxonomic guide to the polychaetes of the northern Gulf of Mexico. Volume III. Final report to the Minerals Management Service. J. M. Uebelacker and P. G. Johnson. Mobile, AL, Barry A. Vittor & Associates, Inc. (1984). Taxonomic guide to the polychaetes of the northern Gulf of Mexico. -

Marine Soiences Centre Zooplankton Distribution in the Arctio Ooean
---------.. - -_.---_.----------- ----- - ---- ----_._ ..... Gareth C.H. Harding Marine Soiences Centre Zooplankton Distribution in the Arctio Ooean " ,f;" .j ZOOPLANKTON DISTRIBUTION IN THE ARCTIC OCEAN WITH NOTES ON LIFE CYCLES by Gareth C.H. Harding • A thesis submitted to the Faculty of Graduate Studies and Research in partial fulfilment of the requirements for the degree of Master of Science. ÎI'Iarine Sciences Centre, McGill University, August 1966. Montreal. ® Gareth C .H. Harding 1967 .' - ACKNOTJJLEDGEMENTS I ~'lould like to thank Dr. IvI.J • Dunbar, under whose guidance this work was undertaken, Dr. I.A.McLaren, Dr. E.H.Grainger, of the Fisheries Research Board of Canada, Arctic Unit, and other members of the IvIcGill ~mrine Sciences Centre for their assistance in and discussion of ~ this thesis. I should like to thank IvIrs. D. IvIeClellan for h~r help and also for introducing meto the taxonomy of copepods. The work was made possible by the support of the Office of Naval Research and the staff of the Aretie Research Laboratory, Point Barrow. Here I should like to thank in particular Dr. M. Britton and Dr. M. Brewer. I would also like to thank the field team from Lamont Geological Observatory on T-3; through mutual co operation both parties managedto collect oceanographie data for both teams throughout the summer, despite the break-down of the l\ïcGill winch and the aecidental short- age of manpower on the Lamont side. I would also like to thank my partner and good companion in the field-work, 1>1r. IVlartin Weinstein. O.N.R. funds made this work possible. -
Distributional Records of Ross Sea (Antarctica) Planktic
ZooKeys 969: 1–22 (2020) A peer-reviewed open-access journal doi: 10.3897/zookeys.969.52334 DATA PAPER https://zookeys.pensoft.net Launched to accelerate biodiversity research Distributional records of Ross Sea (Antarctica) planktic Copepoda from bibliographic data and samples curated at the Italian National Antarctic Museum (MNA): checklist of species collected in the Ross Sea sector from 1987 to 1995 Guido Bonello1, Marco Grillo1, Matteo Cecchetto1,2, Marina Giallain2, Antonia Granata3, Letterio Guglielmo4, Luigi Pane2, Stefano Schiaparelli1,2 1 Italian National Antarctic Museum (MNA, Section of Genoa), University of Genoa, Genoa, Italy 2 Depart- ment of Earth, Environmental and Life Science (DISTAV), University of Genoa, Genoa, Italy 3 Department of Chemical, Biological, Pharmaceutical and Environmental Sciences (ChiBioFarAm), University of Messina, Messina, Italy 4 Stazione Zoologica Anton Dohrn (SZN), Villa Pace, Messina, Italy Corresponding author: Stefano Schiaparelli ([email protected]) Academic editor: Kai Horst George | Received 23 March 2020 | Accepted 9 July 2020 | Published 17 September 2020 http://zoobank.org/AE10EA51-998A-43D5-9F5B-62AE23B31C14 Citation: Bonello G, Grillo M, Cecchetto M, Giallain M, Granata A, Guglielmo L, Pane L, Schiaparelli S (2020) Distributional records of Ross Sea (Antarctica) planktic Copepoda from bibliographic data and samples curated at the Italian National Antarctic Museum (MNA): checklist of species collected in the Ross Sea sector from 1987 to 1995. ZooKeys 969: 1–22. https://doi.org/10.3897/zookeys.969.52334 Abstract Distributional data on planktic copepods (Crustacea, Copepoda) collected in the framework of the IIIrd, Vth, and Xth Expeditions of the Italian National Antarctic Program (PNRA) to the Ross Sea sector from 1987 to 1995 are here provided.