Revising the Definition of the Crustacean Seta and Setal
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Effects of Temperature on Seta Elongation in Atrichum Undulatum^ 2- 3
EFFECTS OF TEMPERATURE ON SETA ELONGATION IN ATRICHUM UNDULATUM^ 2- 3 DENNIS WM. STEVENSON4, JAMES R. RASTORFER, AND RAY E. SHOWMAN Department of Botany, The Ohio State University, Columbus, Ohio 43210 ABSTRACT Field-collected gametophytes of Atrichum undulatum were placed in two growth chambers which were maintained at the same light intensity and light period, but at two different temperature regimes. After sporophyte development, differences in seta lengths were observed. Measurements of cell lengths revealed that attached setae grown in the high-temperature regime (12°-22°C) were longer than those grown in a low-temperature regime (3°-12°C), as a result of both more cell divisions and a larger average cell length. Thus, temperature appeared to influence both cell division and cell elongation in the setae of Atrichum undulatum. INTRODUCTION Sporophytes of most liverworts and mosses (Bryophyta) consist of a basal foot attached to the gametophyte and a seta or stalk, which supports a spore-bearing capsule at its apex. Lengths of setae at sporophyte maturity differ among species, varying from a minute structure to a conspicuous organ which may exceed 5 cm (Watson, 1964). Setae of the Common Hair-Cap Moss (Polytrichum commune) are reported to vary from 6 to 12 cm in length (Welch, 1957), but unfortunately it is not clearly understood whether these differences are caused by genetic factors or environmental factors or both. A few studies have suggested that seta elonga- tion can apparently be influenced by environmental factors (e.g. temperature; light intensity; day length), and that there may be interactions of internal plant factors (e.g. -

Ocean Deoxygenation and Copepods: Coping with Oxygen Minimum Zone Variability
Biogeosciences, 17, 2315–2339, 2020 https://doi.org/10.5194/bg-17-2315-2020 © Author(s) 2020. This work is distributed under the Creative Commons Attribution 4.0 License. Ocean deoxygenation and copepods: coping with oxygen minimum zone variability Karen F. Wishner1, Brad Seibel2, and Dawn Outram1 1Graduate School of Oceanography, University of Rhode Island, Narragansett, RI 02882, USA 2College of Marine Science, University of South Florida, St. Petersburg, FL 33701, USA Correspondence: Karen F. Wishner ([email protected]) Received: 27 September 2019 – Discussion started: 28 October 2019 Revised: 31 March 2020 – Accepted: 2 April 2020 – Published: 24 April 2020 Abstract. Increasing deoxygenation (loss of oxygen) of compression concept). These distribution depths changed by the ocean, including expansion of oxygen minimum zones tens to hundreds of meters depending on the species, oxygen (OMZs), is a potentially important consequence of global profile, and phenomenon. For example, at the lower oxycline, warming. We examined present-day variability of vertical the depth of maximum abundance for Lucicutia hulsemannae distributions of 23 calanoid copepod species in the East- shifted from ∼ 600 to ∼ 800 m, and the depth of diapause for ern Tropical North Pacific (ETNP) living in locations with Eucalanus inermis shifted from ∼ 500 to ∼ 775 m, in an ex- different water column oxygen profiles and OMZ inten- panded OMZ compared to a thinner OMZ, but remained at sity (lowest oxygen concentration and its vertical extent in similar low oxygen levels in -

Tesis Estructura Comunitaria De Copepodos .Pdf
Universidad de Concepción Dirección de Postgrado Facultad de Ciencias Naturales y Oceanográficas Programa de Magister en Ciencias mención Oceanografía Estructura comunitaria de copépodos pelágicos asociados a montes submarinos de la Dorsal Juan Fernández (32-34°S) en el Pacífico Sur Oriental Tesis para optar al grado de Magíster en Ciencias con mención en Oceanografía PAMELA ANDREA FIERRO GONZÁLEZ CONCEPCIÓN-CHILE 2019 Profesora Guía: Pamela Hidalgo Díaz Departamento de Oceanografía, Facultad de Ciencias Naturales y Oceanográficas Universidad de Concepción Profesor Co-guía: Rubén Escribano Departamento de Oceanografía, Facultad de Ciencias Naturales y Oceanográficas Universidad de Concepción La Tesis de “Magister en Ciencias con mención en Oceanografía” titulada “Estructura comunitaria de copépodos pelágicos asociados a montes submarinos de la Dorsal Juan Fernández (32-34°S) en el Pacífico sur oriental”, de la Srta. “PAMELA ANDREA FIERRO GONZÁLEZ” y realizada bajo la Facultad de Ciencias Naturales y Oceanográficas, Universidad de Concepción, ha sido aprobada por la siguiente Comisión de Evaluación: Dra. Pamela Hidalgo Díaz Profesora Guía Universidad de Concepción Dr. Rubén Escribano Profesor Co-Guía Universidad de Concepción Dr. Samuel Hormazábal Miembro de la Comisión Evaluadora Pontificia Universidad Católica de Valparaíso Dr. Fabián Tapia Director Programa de Magister en Oceanografía Universidad de Concepción ii A Juan Carlos y Sebastián iii AGRADECIMIENTOS Agradezco a quienes con su colaboración y apoyo hicieron posible el desarrollo y término de esta tesis. En primer lugar, agradezco a los miembros de mi comisión de tesis. A mi profesora guía, Dra. Pamela Hidalgo, por apoyarme y guiarme en este largo camino de formación académica, por su gran calidad humana, contención y apoyo personal. -

Faunistic and Biological Notes on Marine Invertebrates Iii
Biologiske Meddelelser udgivet af Det Kongelige Danske Videnskabernes Selskab Bind 23, no. 1 Biol. Medd. Dan. Vid. Selsk. 23, no. 1 (1956) FAUNISTIC AND BIOLOGICAL NOTES ON MARINE INVERTEBRATES III. • The Reproduction and Larval Development of some Polychaetes from the Isefjord, with some Faunistic Notes. B Y • ERIK RASMUSSEN (Report from the Isefjord Laboratory No. 3) København 1956 i kommission hos Ejnar Munksgaard D et Kongelige Danske V idenskabernes Selskab udgiver følgende publikationsrækker : L'Académie Royale des Sciences et des Lettres de Danemark publie les séries suivantes: Bibliograûsk forkortelse Ahréi/iation bibliographique Oversigt over selskabets virksomhed (8°) Overs. Dan. Vid. Selsk. (Annuaire) Historisk-filologiske Meddelelser (8°) Hist. Filol. Medd. Dan. Vid. Selsk. -Historisk-filologiske Skrifter (4°) Hist. Filol. Skr. Dan. Vid. Selsk. (Histoire et Philologie) Arkæologisk-kunsthistoriske Meddelelser (8°) Arkæol. Kunsthist. Medd. Dan. Vid. Selsk. Arkæologisk-kunsthistoriske Skrifter (4°) Arkæol. Kunsthist. Skr. Dan. Vid. (Archéologie et Histoire de I’Art} Selsk. Filosofiske Meddelelser (8°) Filos. Medd. Dan. Vid. Selsk. (Philosophie) Matematisk-fysiske Meddelelser (8°) Mat. Fys. Medd. Dan. Vid. Selsk. (Mathémaliqnes et Physique) Biologiske Meddelelser (8°) Biol. Medd. Dan. Vid. Selsk. Biologiske Skrifter (4®) Biol. Skr. Dan. Vid. Selsk. (Biologie) Selskabets sekretariat og postadresse: Dantes plads 5, København V. L'adresse poslale du secrétariat de VAcadémie est: Det Kongelige Danske Videnskabernes Selskab, Dantes plads 5, København V, Danmark. Selskabets kommissionær: Ejnar Munksgaard’s forlag, Nørregade 6, København K. Les publications sont en vente chez le commissionnaire: Ejnar Münksgaard, éditeur. Nørregade 6, København K, Danmark. Biologiske Meddelelser udgivet af Det Kongelige Danske Videnskabernes Selskab Bind 23, no. 1 Biol. Medd. Dan. -

OREGON ESTUARINE INVERTEBRATES an Illustrated Guide to the Common and Important Invertebrate Animals
OREGON ESTUARINE INVERTEBRATES An Illustrated Guide to the Common and Important Invertebrate Animals By Paul Rudy, Jr. Lynn Hay Rudy Oregon Institute of Marine Biology University of Oregon Charleston, Oregon 97420 Contract No. 79-111 Project Officer Jay F. Watson U.S. Fish and Wildlife Service 500 N.E. Multnomah Street Portland, Oregon 97232 Performed for National Coastal Ecosystems Team Office of Biological Services Fish and Wildlife Service U.S. Department of Interior Washington, D.C. 20240 Table of Contents Introduction CNIDARIA Hydrozoa Aequorea aequorea ................................................................ 6 Obelia longissima .................................................................. 8 Polyorchis penicillatus 10 Tubularia crocea ................................................................. 12 Anthozoa Anthopleura artemisia ................................. 14 Anthopleura elegantissima .................................................. 16 Haliplanella luciae .................................................................. 18 Nematostella vectensis ......................................................... 20 Metridium senile .................................................................... 22 NEMERTEA Amphiporus imparispinosus ................................................ 24 Carinoma mutabilis ................................................................ 26 Cerebratulus californiensis .................................................. 28 Lineus ruber ......................................................................... -

Molecular Species Delimitation and Biogeography of Canadian Marine Planktonic Crustaceans
Molecular Species Delimitation and Biogeography of Canadian Marine Planktonic Crustaceans by Robert George Young A Thesis presented to The University of Guelph In partial fulfilment of requirements for the degree of Doctor of Philosophy in Integrative Biology Guelph, Ontario, Canada © Robert George Young, March, 2016 ABSTRACT MOLECULAR SPECIES DELIMITATION AND BIOGEOGRAPHY OF CANADIAN MARINE PLANKTONIC CRUSTACEANS Robert George Young Advisors: University of Guelph, 2016 Dr. Sarah Adamowicz Dr. Cathryn Abbott Zooplankton are a major component of the marine environment in both diversity and biomass and are a crucial source of nutrients for organisms at higher trophic levels. Unfortunately, marine zooplankton biodiversity is not well known because of difficult morphological identifications and lack of taxonomic experts for many groups. In addition, the large taxonomic diversity present in plankton and low sampling coverage pose challenges in obtaining a better understanding of true zooplankton diversity. Molecular identification tools, like DNA barcoding, have been successfully used to identify marine planktonic specimens to a species. However, the behaviour of methods for specimen identification and species delimitation remain untested for taxonomically diverse and widely-distributed marine zooplanktonic groups. Using Canadian marine planktonic crustacean collections, I generated a multi-gene data set including COI-5P and 18S-V4 molecular markers of morphologically-identified Copepoda and Thecostraca (Multicrustacea: Hexanauplia) species. I used this data set to assess generalities in the genetic divergence patterns and to determine if a barcode gap exists separating interspecific and intraspecific molecular divergences, which can reliably delimit specimens into species. I then used this information to evaluate the North Pacific, Arctic, and North Atlantic biogeography of marine Calanoida (Hexanauplia: Copepoda) plankton. -

ADHESIVE FORCE of a SINGLE GECKO SETA a Thesis Presented
ADHESIVE FORCE OF A SINGLE GECKO SETA A Thesis Presented to The Graduate Faculty of The University of Akron In Partial Fulfillment of the Requirements for the Degree Master of Science Lan Yu May, 2018 ADHESIVE FORCE OF A SINGLE GECKO SETA Lan Yu Thesis Approved: Accepted: Advisor Dean of College Dr. Ali Dhinojwala Dr. Eric J. Amis Committee Member Dean of the Graduate School Dr. Hunter King Dr. Chand K. Midha Department Chair Date Dr. Coleen Pugh ii ABSTRACT The gecko’s adhesion system uses arrays of setae to achieve strong and repeatable adhesion. Observation of the toe pad structure shows that shorter setae are generally proximal while longer ones distal [39]. We hypothesized that a seta of longer length would generate higher adhesive force, as long and slender setae have lower bending modulus, and therefore making it easier to contact spatulae with the surface. By following previous single seta experiments [11], we first measured the shear force generated by an isolated gecko seta using Nano Bionix. We also tested the length hypothesis by measuring the shear adhesion ability of single seta of varying lengths ranging from 80 to 140µm, which was taken from different parts of the gecko toe pad from distal to proximal. Measurements gave the result of shear forces of a single seta in the range of 80 to 250µN and an average of 136.81µN. This number is lower than the average value 194µN in previous studies, which is tested under a preload of 15µN [11]. In addition, results from 12 individual setae suggested that shear adhesion force of a single seta is independent of setal length and dependent on setal diameter. -

Observing Copepods Through a Genomic Lens James E Bron1*, Dagmar Frisch2, Erica Goetze3, Stewart C Johnson4, Carol Eunmi Lee5 and Grace a Wyngaard6
Bron et al. Frontiers in Zoology 2011, 8:22 http://www.frontiersinzoology.com/content/8/1/22 DEBATE Open Access Observing copepods through a genomic lens James E Bron1*, Dagmar Frisch2, Erica Goetze3, Stewart C Johnson4, Carol Eunmi Lee5 and Grace A Wyngaard6 Abstract Background: Copepods outnumber every other multicellular animal group. They are critical components of the world’s freshwater and marine ecosystems, sensitive indicators of local and global climate change, key ecosystem service providers, parasites and predators of economically important aquatic animals and potential vectors of waterborne disease. Copepods sustain the world fisheries that nourish and support human populations. Although genomic tools have transformed many areas of biological and biomedical research, their power to elucidate aspects of the biology, behavior and ecology of copepods has only recently begun to be exploited. Discussion: The extraordinary biological and ecological diversity of the subclass Copepoda provides both unique advantages for addressing key problems in aquatic systems and formidable challenges for developing a focused genomics strategy. This article provides an overview of genomic studies of copepods and discusses strategies for using genomics tools to address key questions at levels extending from individuals to ecosystems. Genomics can, for instance, help to decipher patterns of genome evolution such as those that occur during transitions from free living to symbiotic and parasitic lifestyles and can assist in the identification of genetic mechanisms and accompanying physiological changes associated with adaptation to new or physiologically challenging environments. The adaptive significance of the diversity in genome size and unique mechanisms of genome reorganization during development could similarly be explored. -

Plant Reproduction
AccessScience from McGraw-Hill Education Page 1 of 10 www.accessscience.com Plant reproduction Contributed by: Scott D. Russell Publication year: 2014 The formation of a new plant that is either an exact copy or recombination of the genetic makeup of its parents. There are three types of plant reproduction considered here: (1) vegetative reproduction, in which a vegetative organ forms a clone of the parent; (2) asexual reproduction, in which reproductive components undergo a nonsexual form of production of offspring without genetic rearrangement, also known as apomixis; and (3) sexual reproduction, in which meiosis (reduction division) leads to formation of male and female gametes that combine through syngamy (union of gametes) to produce offspring. See also: PLANT; PLANT PHYSIOLOGY. Vegetative reproduction Unlike animals, plants may be readily stimulated to produce identical copies of themselves through cloning. In animals, only a few cells, which are regarded as stem cells, are capable of generating cell lineages, organs, or new organisms. In contrast, plants generate or produce stem cells from many plant cells of the root, stem, or leaf that are not part of an obvious generative lineage—a characteristic that has been known as totipotency, or the general ability of a single cell to regenerate a whole new plant. This ability to establish new plants from one or more cells is the foundation of plant biotechnology. In biotechnology, a single cell may be used to regenerate new organisms that may or may not genetically differ from the original organism. If it is identical to the parent, it is a clone; however, if this plant has been altered through molecular biology, it is known as a genetically modified organism (GMO). -
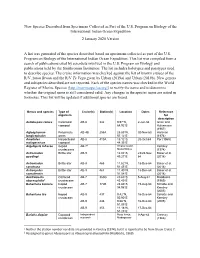
New Species Described from Specimens Collected As Part of the U.S
New Species Described from Specimens Collected as Part of the U.S. Program on Biology of the International Indian Ocean Expedition 2 January 2020 Version A list was generated of the species described based on specimens collected as part of the U.S. Program on Biology of the International Indian Ocean Expedition. This list was compiled from a search of publications cited by scientists involved in the U.S. Program on Biology and publications held by the Smithsonian Institution. The list includes holotypes and paratypes used to describe species. The cruise information was checked against the list of known cruises of the R/V Anton Bruun and the R/V Te Vega given by Urban (2019a) and Urban (2019b). New genera and subspecies described are not reported. Each of the species names was checked in the World Register of Marine Species (http://marinespecies.org/) to verify the name and to determine whether the original name is still considered valid. Any changes in the species name are noted in footnotes. This list will be updated if additional species are found. Genus and species Type of Cruise(s) Station(s) Location Dates Reference organism for description Aetideopsis retusa Calanaoid AB-6 342 9.97°S, 2-Jun-64 Grice and copepod 64.92°E Hulsemann (1967) Aglaophamus Polychaete AB-4B 255A 25.83°N, 30-Nov-63 Hartman longicephalus worm 57.12°E (1974) Ameliotes Harpacticoid AB-8 410A 15.12°S, 20-Oct-64 Por (1969) malagassicus copepod 44.35°E Argeiopsis inhacae isopod AB-71 Inhaca Island, Kensley crustaceans Mozambique (1974) Astrocladus Brittle star AB-9 12.83°S, 23/26-Nov- Baker et al. -

Four New Species of Heteromysis (Crustacea: Mysida) from Public Aquaria in Hawaii, Florida, and Western to Central Europe
European Journal of Taxonomy 735: 133–175 ISSN 2118-9773 https://doi.org/10.5852/ejt.2021.735.1247 www.europeanjournaloftaxonomy.eu 2021 · Wittmann K.J. & Abed-Navandi D. This work is licensed under a Creative Commons Attribution License (CC BY 4.0). Research article urn:lsid:zoobank.org:pub:F1CE3697-319D-4D02-A99F-11A0E16A8743 Four new species of Heteromysis (Crustacea: Mysida) from public aquaria in Hawaii, Florida, and Western to Central Europe Karl J. WITTMANN 1,* & Daniel ABED-NAVANDI 2 1 Medical University of Vienna, Department of Environmental Health, Kinderspitalgasse 15, 1090 Vienna, Austria. 2 Haus des Meeres – Aqua Terra Zoo, Fritz Grünbaum Platz 1, 1060 Vienna, Austria. * Corresponding author: [email protected] 2 Email: [email protected] 1 urn:lsid:zoobank.org:author:C90E7BC4-A27A-4B41-93F3-6224D17795FF 2 urn:lsid:zoobank.org:author:179B83B0-8C8B-4DF5-8986-4227B8E1BB9B Abstract. Four new species of the subgenus Heteromysis (Olivemysis) were detected in material from (sub)-tropical aquaria in six public aquarium institutions around the globe. Modifications of pleopods by spines represent the strongest structural complex used for differentiation within this subgenus: male pleopods 1–4 modified in H. smithsoniana sp. nov., male pleopods 2–4 plus female pleopod 2 in H. hornimani sp. nov. and H. waikikensis sp. nov. Additional important diagnostic characters are provided by the antennulae, uropods, and telson. The male of H. sixi sp. nov. represents a very rare case within the genus Heteromysis by having only pleopod 2 modified by flagellate spines. The definition of the subgenus Olivemysis is modified in order to include H. -

Trabajo De Grado Para Optar Al Título De Biólogo Marino
ESTRUCTURA DEL ZOOPLANCTON Y SU RELACIÓN CON LAS CONDICIONES MARINAS EN EL CARIBE NORTE COLOMBIANO LEIDY JOHANNA HERNÁNDEZ RIVERA Tesis de maestría para optar al título de Magíster en Ciencias Marinas Director ADOLFO SANJUAN MUÑOZ Profesor Asociado Biólogo Marino Magíster en Gestión Ambiental en Zonas Costeras Máster en Biodiversidad Animal Codirector ANDRÉS FRANCO HERRERA Director Departamento de Ciencias Biológicas y Ambientales Biólogo Marino Doctor en Oceanografía PROGRAMA DE MAESTRÍA EN CIENCIAS MARINAS DEPARTAMENTO DE CIENCIAS BIOLÓGICAS Y AMBIENTALES FACULTAD DE CIENCIAS NATURALES E INGENIERÍA UNIVERSIDAD DE BOGOTÁ JORGE TADEO LOZANO SANTA MARTA 2019 ESTRUCTURA DEL ZOOPLANCTON Y SU RELACIÓN CON LAS CONDICIONES MARINAS EN EL CARIBE NORTE COLOMBIANO Hernández Rivera, L. Programa de Maestría en Ciencias Marinas. Departamento de ciencias biológicas y ambientales. facultad de Ciencias Naturales e Ingeniería. Universidad de Bogotá Jorge Tadeo Lozano. RESUMEN Con el objetivo de determinar la estructura en términos de abundancia, biomasa, composición y diversidad del ensamblaje zooplanctónico en la costa del Caribe norte colombiano y relacionarla con las condiciones del agua, se llevaron a cabo dos cruceros de investigación en las dos épocas climáticas típicas de la región (seca y lluviosa). Las estaciones fueron ubicadas en dos de las provincias fisicoquímicas determinadas en el Caribe colombiano; grupo 2: río Magdalena-CGSM (región 1) y grupo 6: PNN Tayrona-Guajira (región 2). Estos se realizaron sobre una grilla de 16 estaciones, donde se efectuaron arrastres circulares superficiales. Se encontraron 87 familias-morfotipos encontrándose la mayor riqueza de familias en la región 1 en la época seca, mientras que las mayores contribuciones en abundancias y biomasas se obtuvieron en época lluviosa en la región PNN Tayrona-Guajira.