2020 AAZV Proceedings.Pdf
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
WINTER 2015/2016! This Guide Gets Bigger and Better Every Year! We’Ve Packed This Year’S Winter Excitement Guide with Even More Events and Festivals
WELCOME TO WINTER 2015/2016! This guide gets bigger and better every year! We’ve packed this year’s Winter Excitement Guide with even more events and festivals. But keep your toque-covered ear to the ground for the spontaneous events that happen, like last year’s awesome #yegsnowfight We’re all working together, as a community, to think differently, to embrace the beauty of our snowy season, and to make Edmonton a great winter city. Edmonton’s community-led, award-winning WinterCity Strategy is our roadmap for reaching greatness. We are truly proud to say that we are on our way to realizing all the great potential our winters have to offer. New for this winter, we’ve got a blog for sharing ideas and experiences! Check it out at www.wintercityedmonton.ca If you haven’t joined us on Facebook and Twitter yet, we invite you to join the conversation. Let us know how you celebrate winter and be a part of the growing community that’s making Edmonton a great place to live, work and play in the wintertime. Now get out there and have some wintry fun! www.edmonton.ca/wintercitystrategy Facebook.com/WinterCityEdmonton @WinterCityYEG / #wintercityyeg Edmonton Ski Club Winter Warm-up Fundraiser Saturday, Oct 3, 2015 Edmonton Ski Club (9613 – 96 Avenue) www.edmontonskiclub.com Start winter with the ESC Winter Warm-up Fundraiser! Join us for a pig roast and family games. Visit our website for more details. International Walk to School Week (iWALK) Oct 5 – 9, 2015 www.shapeab.com iWALK is part of the Active & Safe Routes to School Program, promoting active travel to school! You can register online. -

2018 Annual Progress Report Reporting Period January-December 2018
2018 Annual Progress Report Reporting Period January-December 2018 By Dr Laurie Marker Executive Director Cheetah Conservation Fund P.O. Box 1755 Otjiwarongo, Namibia Phone: 067 306225 Fax: 067 306247 Email: [email protected] Internal Use Only 1 Table Of Contents I. Executive Summary 4 II. Organisational Structure 6 III. Research 7 A. POPULATION DYNAMICS 7 B. MEDICAL EXAMINATIONS 8 1. EXAMINATIONS UNDER ANAESTHESIA 8 2. EXAMINATIONS WITHOUT ANAESTHESIA 9 3. HEALTH-RELATED MEDICAL EXAMINATIONS: CAPTIVE CHEETAHS 10 4. DENTAL PROCEDURES ON CCF’S WILD AND CAPTIVE CHEETAHS 12 5. RELEASED CHEETAH EXAMINATIONS 12 6. WILD CHEETAH EXAMINATIONS 13 7. DEATHS, EUTHANASIA, AND NECROPSIES 13 8. NON-CHEETAH CARNIVORE EXAMINATIONS AND NECROPSIES 14 C. HEALTH AND REPRODUCTION 16 1. GENOME RESOURCE BANK 16 D. CONSERVATION GENETICS 16 1. LIFE TECHNOLOGIES CONSERVATION GENETICS LABORATORY 16 2. SCAT DETECTION DOGS 20 E. LARGE CARNIVORE RESEARCH AND ECOLOGY 23 1. GO GREEN PROJECT – CARNIVORE LANDSCAPE DISTRIBUTION AND ABUNDANCE 23 2. PILOT PROJECTS: E-SHEPHERD COLLARS AND FOXLIGHTS 32 3. CHEETAH RELEASES AND MONITORING 46 F. ECOSYSTEM RESEARCH 54 1. WEATHER MONITORING 54 2. GAME MONITORING 55 3. BUSH ENCROACHMENT AND BIODIVERSITY 62 4. CHEETAH/LEOPARD CAMERA TRAP STUDY 63 5. GIRAFFE IDENTIFICATION 64 6. CCF RHINO RESERVE 66 7. VISITING RESEARCHERS 66 G. SCIENTIFIC PUBLICATIONS AND PAPERS 67 1. BOOKS 67 2. BOOK CHAPTERS 67 3. PAPERS 70 4. SUBMITTED PAPERS 70 5. PAPERS IN PREPARATION 70 IV. Conservation 71 A. LIVESTOCK GUARDING DOG PROGRAMME 71 1. PROGRAMME OVERVIEW 71 2. BREEDING AND PUPPY PLACEMENTS 72 3. FOLLOW-UP ON PRIOR PLACEMENTS AND HEALTH SURVEY 74 4. -

National Resource Material Green and Black Poison Frog (Dendrobates Auratus)
Indicative 10 Project National Resource Material Green and Black Poison frog (Dendrobates auratus) Michelle T. Christy and Win Kirkpatrick 2017 Department of Primary Industries and Regional Development 3 Baron-Hay Court, South Perth, WA 6151 An Invasive Animals CRC Project Contents Summary ............................................................................. 2 Key Messages ................................................................... 2 Classification ................................................................... 2 Common names ................................................................ 3 Biology and Ecology ................................................................ 3 Identification ................................................................... 3 Behaviours and Traits ......................................................... 4 Food and Foraging ............................................................. 4 Reproduction and Lifecycle ................................................. 5 Habitat ......................................................................... 5 Global Range ........................................................................ 5 Potential for Introduction ........................................................ 6 Potential for Eradication.......................................................... 7 Impacts ............................................................................... 7 Economic ........................................................................ 7 Environmental -
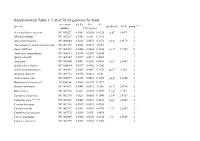
Supplementary Table 1: List of 76 Mt Genomes for Birds
Supplementary Table 1: List of 76 mt genomes for birds. accession Ka/Ks Ka Ks species speed(m/s) Ln S group * * number 13 mt genes Acrocephalus scirpaceus NC 010227 0.0464 0.0288 0.6228 6.6d 1.8871 2 Alectura lathami NC 007227 0.0343 0.032 0.9311 2 Anas platyrhynchos NC 009684 0.0252 0.0073 0.2873 18.5a 2.9178 2 Anomalopteryx didiformis (die out) NC 002779 0.0508 0.0027 0.054 1 Anser albifrons NC 004539 0.0468 0.0088 0.1856 16.1a 2.7788 2 Anseranas semipalmata NC 005933 0.0364 0.0207 0.5628 2 Apteryx haastii NC 002782 0.0599 0.0273 0.4561 1 Apus apus NC 008540 0.0401 0.0241 0.6431 10.6a 2.3609 2 Archilochus colubris NC 010094 0.0397 0.0382 0.9242 2 Ardea novaehollandiae NC 008551 0.0423 0.0067 0.1552 10.2c# 2.322 2 Arenaria interpres NC 003712 0.0378 0.0213 0.581 2 Aythya americana NC 000877 0.0309 0.0092 0.2987 24.4b 3.1946 2 Bambusicola thoracica* EU165706 0.0503 0.0144 0.2872 1 Branta canadensis NC 007011 0.0444 0.0092 0.206 16.7a 2.8154 2 Buteo buteo NC 003128 0.049 0.0167 0.3337 11.6a 2.451 2 Casuarius casuarius NC 002778 0.028 0.0085 0.3048 13.9f 2.6319 2 Cathartes aura * * * * NC 007628 0.0468 0.0239 0.4676 10.6c 2.3609 2 Ciconia boyciana NC 002196 0.0539 0.0031 0.0563 2 Ciconia ciconia NC 002197 0.0501 0.0029 0.0572 9.1d 2.2083 2 Cnemotriccus fuscatus NC 007975 0.0369 0.038 1.0476 2 Corvus frugilegus NC 002069 0.0428 0.0298 0.6526 13a 2.5649 2 Coturnix chinensis NC 004575 0.0325 0.0122 0.3762 2 Coturnix japonica NC 003408 0.0432 0.0128 0.2962 2 Cygnus columbianus NC 007691 0.0327 0.0089 0.2702 18.5a 2.9178 2 Dinornis giganteus -
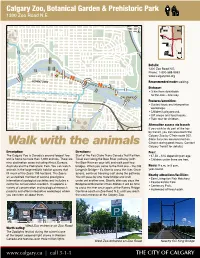
Calgary Zoo Commute
Calgary Zoo, Botanical Garden & Prehistoric Park 1300 Zoo Road N.E. ± NW NE TRANS CENTRE ST CANADA BO W HWY RI VER MEMORIAL DR TR MACLEOD SW SE 2" Details: 1300 Zoo Road N.E. ! ! ! Phone: 1-800-588-9993 ! ! ! 1 www.calgaryzoo.org ! .1 ! ! ! DOWNTOWN ! ! ! ! ! Recommended mode: walking. ! ! ! 1.3 ! ! ! 2" ! ! ! ! Distance: ! ! 2" 2" 2" 2" 2" • 3 km from downtown 2" 2" 2" ! 2" 2" 2" 2" to the Zoo – one way. CALGARY ZOO Features/amenities: • Guided tours and interpretive workshops. • Children’s playground. • Gift shops and food kiosks. 2" • Train tour for children. Alternative access via transit: If you wish to do part of the trip by transit, you can also reach the Calgary Zoo by CTrain route 202. (Note: bicycles are restricted on Walk with the animals CTrains during peak hours. Contact Calgary Transit for details.) Description: Directions: Fees: The Calgary Zoo is Canada’s second largest zoo Start at the Eau Claire Trans Canada Trail Pavilion. • $7.50 – $16 depending on age. and is home to more than 1,000 animals. There are Travel east along the Bow River pathway (with • Children under three are free. nine destination areas including Africa, Eurasia, the Bow River on your left) and walk past two Australia and the Prehistoric Park. You can watch bridges. When you come to the third one – the Old Hours: 9 a.m. to 5 p.m. animals in the large realistic habitat spaces that Langevin Bridge – it’s time to cross the river. Once year-round. fill much of the Zoo’s 159 hectares. -

2020 Annual Report
2020 ANNUAL REPORT Providing a level of excellence that makes the Rosamond Gifford Zoo a national leader in animal care, conservation and visitor experience. 1 A JOINT MESSAGE FROM THE EXECUTIVE DIRECTOR AND THE CHAIR OF THE BOARD TABLE OF CONTENTS Facebook followers increased from 61.3K at the start The year 2020 was undoubtedly the most challenging in our history. However, we can celebrate of 2020 to 65.8K on many successes which proved that perseverance, teamwork and, most importantly, a supportive December 31, 2020, adding community can see us through anything. Navigating a Pandemic 4 followers. Over the past year, our amazing Friends of the Zoo community truly went above and beyond for 4,500 Maintaining Partnerships your zoo. You let us know how much you missed visiting while we were closed, you came back 9 Surpassed as soon as you could, and you contributed to several campaigns to help the zoo recover from the 10 Engaging our Community pandemic. 25,000 Capital Improvements followers on Instagram, When we substituted a fundraising campaign - $50K for 50 Years – for a Friends of the Zoo 50th 12 a huge milestone. anniversary celebration, you pitched in to help us raise more than $20,000 over our $50,000 goal. When we offered a two-month extension on memberships to cover the COVID closure, most 13 2020 Accomplishments of you donated it back to the zoo. When we asked our volunteers to help the zoo acquire more flamingos to expand our flock, you donated to the Fund for Flamingo Flamboyance. Or, you gave 14 Development and Fundraising to our Annual Appeal on behalf of a baby patas monkey named Iniko -- “born during troubled Nearly 9,140 times.” 15 New Leadership children and adults actively participated in conservation education learning programs When, at the end of an already difficult year, we lost our two youngest elephants to another 16 Future Focus deadly virus, you mourned with us, sent messages of encouragement and donated to the Ajay and Batu Memorial Fund to help the new Animal Health Center test for and treat EEHV. -

Engineering the Genome of Minimal Bacteria Using CRISPR/Cas9 Tools Iason Tsarmpopoulos
Engineering the genome of minimal bacteria using CRISPR/Cas9 tools Iason Tsarmpopoulos To cite this version: Iason Tsarmpopoulos. Engineering the genome of minimal bacteria using CRISPR/Cas9 tools. Mi- crobiology and Parasitology. Université de Bordeaux, 2017. English. NNT : 2017BORD0787. tel- 01834971 HAL Id: tel-01834971 https://tel.archives-ouvertes.fr/tel-01834971 Submitted on 11 Jul 2018 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. THÈSE PRÉSENTÉE POUR OBTENIR LE GRADE DE DOCTEUR DE L’UNIVERSITÉ DE BORDEAUX ÉCOLE DOCTORALE Science de la vie et de la Santé SPÉCIALITÉ Microbiologie and Immunologie Par Iason TSARMPOPOULOS Ingénierie de génome de bactéries minimales par des outils CRISPR/Cas9 Sous la direction de : Monsieur Pascal SIRAND-PUGNET Soutenue le jeudi 07 décembre 2017 à 14h00 Lieu : INRA, 71 avenue Edouard Bourlaux 33882 Villenave d'Ornon salle Amphithéâtre Josy et Colette Bové Membres du jury : Mme Cécile BEBEAR Université de Bordeaux et CHU de Bordeaux Président Mme Florence TARDY Anses-Laboratoire de Lyon Rapporteur M. Matthieu JULES Institut Micalis, INRA and AgroParisTech Rapporteur M. David BIKARD Institut Pasteur Examinateur M. Fabien DARFEUILLE INSERM U1212 - CNRS UMR 5320 Invité Mme Carole LARTIGUE-PRAT INRA - Université de Bordeaux Invité M. -

Learning with Wetlands at the Sam Livingston Fish Hatchery: a Marriage of Mind and Nature
University of Calgary PRISM: University of Calgary's Digital Repository Graduate Studies Legacy Theses 1999 Learning with wetlands at the Sam Livingston fish hatchery: A marriage of mind and nature Grieef, Patricia Lynn Grieef, P. L. (1999). Learning with wetlands at the Sam Livingston fish hatchery: A marriage of mind and nature (Unpublished master's thesis). University of Calgary, Calgary, AB. doi:10.11575/PRISM/12963 http://hdl.handle.net/1880/25035 master thesis University of Calgary graduate students retain copyright ownership and moral rights for their thesis. You may use this material in any way that is permitted by the Copyright Act or through licensing that has been assigned to the document. For uses that are not allowable under copyright legislation or licensing, you are required to seek permission. Downloaded from PRISM: https://prism.ucalgary.ca The University of Calgary Leurnhg with wetiads at the Sam Livingston Fish Hatchery: A Marriage of Mind and Nature by Patricia L. Grieef A Master's Degree Project submitted to the Faculty of Environmental Design in partial hlfillment of the requirements for the degree of Master of Environmental Design (Environmental Science) Calgary, Alberta September, 1999 O Patricia L. Grieef, 1999 National Library BibliotWque nationale 1*1 .,&"a& du Canada Acquisitions and Acquisitions et Bibliographic Services services bibliographiques 395 Wellington Street 395. nn, Wellington OttawaON KlAW OCtewaON K1AON4 Canada Canada The author has granted a non- L'auteur a accorde une licence non exclusive licence allowing the exclusive pennettant a la National Library of Canada to Bibliotheque nationale du Canada de reproduce, loan, distribute or sell reproduire, preter, distribuer ou copies of this thesis in microform, vendre des copies de cette these sous paper or electronic formats. -

Supporting Information
Supporting Information Bender et al. 10.1073/pnas.0802779105 Table S1. Species names and calculated numeric values pertaining to Fig. 1 Mitochondrially Nuclear encoded Nuclear encoded Species name encoded methionine, % methionine, % methionine, corrected, % Animals Primates Cebus albifrons 6.76 Cercopithecus aethiops 6.10 Colobus guereza 6.73 Gorilla gorilla 5.46 Homo sapiens 5.49 2.14 2.64 Hylobates lar 5.33 Lemur catta 6.50 Macaca mulatta 5.96 Macaca sylvanus 5.96 Nycticebus coucang 6.07 Pan paniscus 5.46 Pan troglodytes 5.38 Papio hamadryas 5.99 Pongo pygmaeus 5.04 Tarsius bancanus 6.74 Trachypithecus obscurus 6.18 Other mammals Acinonyx jubatus 6.86 Artibeus jamaicensis 6.43 Balaena mysticetus 6.04 Balaenoptera acutorostrata 6.17 Balaenoptera borealis 5.96 Balaenoptera musculus 5.78 Balaenoptera physalus 6.02 Berardius bairdii 6.01 Bos grunniens 7.04 Bos taurus 6.88 2.26 2.70 Canis familiaris 6.54 Capra hircus 6.84 Cavia porcellus 6.41 Ceratotherium simum 6.06 Choloepus didactylus 6.23 Crocidura russula 6.17 Dasypus novemcinctus 7.05 Didelphis virginiana 6.79 Dugong dugon 6.15 Echinops telfairi 6.32 Echinosorex gymnura 6.86 Elephas maximus 6.84 Equus asinus 6.01 Equus caballus 6.07 Erinaceus europaeus 7.34 Eschrichtius robustus 6.15 Eumetopias jubatus 6.77 Felis catus 6.62 Halichoerus grypus 6.46 Hemiechinus auritus 6.83 Herpestes javanicus 6.70 Hippopotamus amphibius 6.50 Hyperoodon ampullatus 6.04 Inia geoffrensis 6.20 Bender et al. www.pnas.org/cgi/content/short/0802779105 1of10 Mitochondrially Nuclear encoded Nuclear encoded Species -

Role of Protein Phosphorylation in Mycoplasma Pneumoniae
Pathogenicity of a minimal organism: Role of protein phosphorylation in Mycoplasma pneumoniae Dissertation zur Erlangung des mathematisch-naturwissenschaftlichen Doktorgrades „Doctor rerum naturalium“ der Georg-August-Universität Göttingen vorgelegt von Sebastian Schmidl aus Bad Hersfeld Göttingen 2010 Mitglieder des Betreuungsausschusses: Referent: Prof. Dr. Jörg Stülke Koreferent: PD Dr. Michael Hoppert Tag der mündlichen Prüfung: 02.11.2010 “Everything should be made as simple as possible, but not simpler.” (Albert Einstein) Danksagung Zunächst möchte ich mich bei Prof. Dr. Jörg Stülke für die Ermöglichung dieser Doktorarbeit bedanken. Nicht zuletzt durch seine freundliche und engagierte Betreuung hat mir die Zeit viel Freude bereitet. Des Weiteren hat er mir alle Freiheiten zur Verwirklichung meiner eigenen Ideen gelassen, was ich sehr zu schätzen weiß. Für die Übernahme des Korreferates danke ich PD Dr. Michael Hoppert sowie Prof. Dr. Heinz Neumann, PD Dr. Boris Görke, PD Dr. Rolf Daniel und Prof. Dr. Botho Bowien für das Mitwirken im Thesis-Komitee. Der Studienstiftung des deutschen Volkes gilt ein besonderer Dank für die finanzielle Unterstützung dieser Arbeit, durch die es mir unter anderem auch möglich war, an Tagungen in fernen Ländern teilzunehmen. Prof. Dr. Michael Hecker und der Gruppe von Dr. Dörte Becher (Universität Greifswald) danke ich für die freundliche Zusammenarbeit bei der Durchführung von zahlreichen Proteomics-Experimenten. Ein ganz besonderer Dank geht dabei an Katrin Gronau, die mich in die Feinheiten der 2D-Gelelektrophorese eingeführt hat. Außerdem möchte ich mich bei Andreas Otto für die zahlreichen Proteinidentifikationen in den letzten Monaten bedanken. Nicht zu vergessen ist auch meine zweite Außenstelle an der Universität in Barcelona. Dr. Maria Lluch-Senar und Dr. -

615.9Barref.Pdf
INDEX Abortifacient, abortifacients bees, wasps, and ants ginkgo, 492 aconite, 737 epinephrine, 963 ginseng, 500 barbados nut, 829 blister beetles goldenseal blister beetles, 972 cantharidin, 974 berberine, 506 blue cohosh, 395 buckeye hawthorn, 512 camphor, 407, 408 ~-escin, 884 hypericum extract, 602-603 cantharides, 974 calamus inky cap and coprine toxicity cantharidin, 974 ~-asarone, 405 coprine, 295 colocynth, 443 camphor, 409-411 ethanol, 296 common oleander, 847, 850 cascara, 416-417 isoxazole-containing mushrooms dogbane, 849-850 catechols, 682 and pantherina syndrome, mistletoe, 794 castor bean 298-302 nutmeg, 67 ricin, 719, 721 jequirity bean and abrin, oduvan, 755 colchicine, 694-896, 698 730-731 pennyroyal, 563-565 clostridium perfringens, 115 jellyfish, 1088 pine thistle, 515 comfrey and other pyrrolizidine Jimsonweed and other belladonna rue, 579 containing plants alkaloids, 779, 781 slangkop, Burke's, red, Transvaal, pyrrolizidine alkaloids, 453 jin bu huan and 857 cyanogenic foods tetrahydropalmatine, 519 tansy, 614 amygdalin, 48 kaffir lily turpentine, 667 cyanogenic glycosides, 45 lycorine,711 yarrow, 624-625 prunasin, 48 kava, 528 yellow bird-of-paradise, 749 daffodils and other emetic bulbs Laetrile", 763 yellow oleander, 854 galanthamine, 704 lavender, 534 yew, 899 dogbane family and cardenolides licorice Abrin,729-731 common oleander, 849 glycyrrhetinic acid, 540 camphor yellow oleander, 855-856 limonene, 639 cinnamomin, 409 domoic acid, 214 rna huang ricin, 409, 723, 730 ephedra alkaloids, 547 ephedra alkaloids, 548 Absorption, xvii erythrosine, 29 ephedrine, 547, 549 aloe vera, 380 garlic mayapple amatoxin-containing mushrooms S-allyl cysteine, 473 podophyllotoxin, 789 amatoxin poisoning, 273-275, gastrointestinal viruses milk thistle 279 viral gastroenteritis, 205 silibinin, 555 aspartame, 24 ginger, 485 mistletoe, 793 Medical Toxicology ofNatural Substances, by Donald G. -

ANNEX 3 ICC-01/09-02/11-67-Anx3 21-04-2011 2/84 EO PT
ICC-01/09-02/11-67-Anx3 21-04-2011 1/84 EO PT No. ICC-01/09-02/11 21-4-11 ANNEX 3 ICC-01/09-02/11-67-Anx3 21-04-2011 2/84 EO PT A PROGRESS REPORT TO THE HON. ATTORNEY-GENERAL BY THE TEAM ON UPDATE OF POST ELECTION VIOLENCE RELATED CASES IN WESTERN, NYANZA, CENTRAL, RIFT-VALLEY, EASTERN, COAST AND NAIROBI PROVINCES MARCH, 2011 NAIROBI ICC-01/09-02/11-67-Anx3 21-04-2011 3/84 EO PT TABLE OF CONTENTS CHAPTER SUBJECT PAGE TRANSMITTAL LETTER IV 1. INTRODUCTION 1 2. GENDER BASED VIOLENCE CASES 7 3. WESTERN PROVINCE 24 3. RIFT VALLEY PROVINCE 30 4. NYANZA PROVINCE 47 5. COAST PROVINCE 62 6. NAIROBI PROVINCE 66 7. CENTRAL PROVINCE 69 8. STATISTICAL ANALYSIS 70 9. CONCLUSION 73 10. APPENDICES ICC-01/09-02/11-67-Anx3 21-04-2011 4/84 EO PT APPENDIX (NO.) LIST OF APPENDICES APP. 1A - Memo from CPP to Hon. Attorney General APP.1B - Memo from CPP to Hon. Attorney General APP.1C - Update on 2007 Post Election Violence offences As at 4th March, 2010 (police commissioner’s report) APP. 1D - Update by Taskforce on Gender Based Violence Cases (police commissioner’s report) APP. 2 - Memo to Solicitor- General from CPP APP. 3 - Letter from PCIO Western APP. 4 - Letter from PCIO Rift Valley APP.5 - Cases Pending Under Investigations in Rift Valley on special interest cases APP.6 - Cases where suspects are known in Rift Valley but have not been arrested APP.7 - Letter from PCIO Nyanza APP.8 - Letter from PCIO Coast APP.9 - Letter from PCIO Nairobi APP.10 - Correspondences from the team ICC-01/09-02/11-67-Anx3 21-04-2011 5/84 EO PT The Hon.