Building Bridges Forpatient CARE
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

———— 2016 Registration Document
———— 2016 REGISTRATION DOCUMENT Including the annual financial report 01 Persons responsible 3 16 Practices of the governing bodies 91 02 Statutory Auditors 5 17 Employees 93 17.1 Employee data 93 03 Selected financial information 7 17.2 Employee share-ownership and profit-sharing 95 04 Risks 11 18 Major shareholders 97 4.1 Risks specific to the Group’s activity 11 18.1 Shareholders and their voting rights 98 4.2 Liquidity risk 14 18.2 Transactions carried out 4.3 Interest rate risk 15 during the year subject to 4.4 Exchange-rate risk 16 Article L. 621-18-2 of the French 4.5 Intangible asset risk 16 Monetary and Financial Code 100 contents 4.6 Environment risk 17 18.3 Share buybacks 101 4.7 Legal and fiscal risks 17 18.4 Market for Altran Technologies securities 102 4.8 Investment risk 17 18.5 Information on the calculation methods 4.9 Brexit risk 18 and effects of adjustments to the conditions covering the subscription or 05 Company information 19 purchase of rights and securities giving 5.1 Company background information and access to the Company’s share capital 103 development 19 18.6 Agreements entered into by the 5.2 Main investments 20 Company which would be amended or terminated upon a change of control of 06 Information about the the Company 103 Group’s businesses 21 18.7 Agreements between shareholders, of 6.1 Core activities 21 which the Company is aware and which may cause restrictions to the transfer of 6.2 The Engineering and R&D Services market 22 shares and/or the exercise of voting rights 104 6.3 Competition 24 18.8 Commitments -

The Epistemology of Evidence in Cognitive Neuroscience1
To appear in In R. Skipper Jr., C. Allen, R. A. Ankeny, C. F. Craver, L. Darden, G. Mikkelson, and R. Richardson (eds.), Philosophy and the Life Sciences: A Reader. Cambridge, MA: MIT Press. The Epistemology of Evidence in Cognitive Neuroscience1 William Bechtel Department of Philosophy and Science Studies University of California, San Diego 1. The Epistemology of Evidence It is no secret that scientists argue. They argue about theories. But even more, they argue about the evidence for theories. Is the evidence itself trustworthy? This is a bit surprising from the perspective of traditional empiricist accounts of scientific methodology according to which the evidence for scientific theories stems from observation, especially observation with the naked eye. These accounts portray the testing of scientific theories as a matter of comparing the predictions of the theory with the data generated by these observations, which are taken to provide an objective link to reality. One lesson philosophers of science have learned in the last 40 years is that even observation with the naked eye is not as epistemically straightforward as was once assumed. What one is able to see depends upon one’s training: a novice looking through a microscope may fail to recognize the neuron and its processes (Hanson, 1958; Kuhn, 1962/1970).2 But a second lesson is only beginning to be appreciated: evidence in science is often not procured through simple observations with the naked eye, but observations mediated by complex instruments and sophisticated research techniques. What is most important, epistemically, about these techniques is that they often radically alter the phenomena under investigation. -
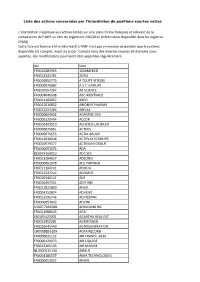
Liste Des Actions Concernées Par L'interdiction De Positions Courtes Nettes
Liste des actions concernées par l'interdiction de positions courtes nettes L’interdiction s’applique aux actions listées sur une plate-forme française et relevant de la compétence de l’AMF au titre du règlement 236/2012 (information disponible dans les registres ESMA). Cette liste est fournie à titre informatif. L'AMF n'est pas en mesure de garantir que le contenu disponible est complet, exact ou à jour. Compte tenu des diverses sources de données sous- jacentes, des modifications pourraient être apportées régulièrement. Isin Nom FR0010285965 1000MERCIS FR0013341781 2CRSI FR0010050773 A TOUTE VITESSE FR0000076887 A.S.T. GROUPE FR0010557264 AB SCIENCE FR0004040608 ABC ARBITRAGE FR0013185857 ABEO FR0012616852 ABIONYX PHARMA FR0012333284 ABIVAX FR0000064602 ACANTHE DEV. FR0000120404 ACCOR FR0010493510 ACHETER-LOUER.FR FR0000076861 ACTEOS FR0000076655 ACTIA GROUP FR0011038348 ACTIPLAY (GROUPE) FR0010979377 ACTIVIUM GROUP FR0000053076 ADA BE0974269012 ADC SIIC FR0013284627 ADEUNIS FR0000062978 ADL PARTNER FR0011184241 ADOCIA FR0013247244 ADOMOS FR0010340141 ADP FR0010457531 ADTHINK FR0012821890 ADUX FR0004152874 ADVENIS FR0013296746 ADVICENNE FR0000053043 ADVINI US00774B2088 AERKOMM INC FR0011908045 AG3I ES0105422002 AGARTHA REAL EST FR0013452281 AGRIPOWER FR0010641449 AGROGENERATION CH0008853209 AGTA RECORD FR0000031122 AIR FRANCE -KLM FR0000120073 AIR LIQUIDE FR0013285103 AIR MARINE NL0000235190 AIRBUS FR0004180537 AKKA TECHNOLOGIES FR0000053027 AKWEL FR0000060402 ALBIOMA FR0013258662 ALD FR0000054652 ALES GROUPE FR0000053324 ALPES (COMPAGNIE) -

Outlook Magazine, Autumn 2018
Washington University School of Medicine Digital Commons@Becker Outlook Magazine Washington University Publications 2018 Outlook Magazine, Autumn 2018 Follow this and additional works at: https://digitalcommons.wustl.edu/outlook Recommended Citation Outlook Magazine, Autumn 2018. Central Administration, Medical Public Affairs. Bernard Becker Medical Library Archives. Washington University School of Medicine, Saint Louis, Missouri. https://digitalcommons.wustl.edu/outlook/188 This Article is brought to you for free and open access by the Washington University Publications at Digital Commons@Becker. It has been accepted for inclusion in Outlook Magazine by an authorized administrator of Digital Commons@Becker. For more information, please contact [email protected]. AUTUMN 2018 Elevating performance BERNARD BECKER MEDICAL LIBRARY BECKER BERNARD outlook.wustl.edu Outlook 3 Atop the Karakoram Mountains, neurologist Marcus Raichle, MD, (center) displays a Mallinckrodt Institute of Radiology banner he created. In 1987, he and other members of a British expedition climbed 18,000 feet above sea level — and then injected radioactive xenon to see how it diffused through their brains. Raichle, 81, has been a central force for decades in the history and science of brain imaging. See page 7. FEATURES MATT MILLER MATT 7 Mysteries explored Pioneering neurologist Marcus Raichle, MD, opened up the human brain to scientific investigation. 14 Growing up transgender The Washington University Transgender Center helps families navigate the complex world of gender identity. COVER Scott Brandon, who sustained a spinal cord injury 16 years ago, said he is 21 Building independence grateful to the Program in Occupational For 100 years, the Program in Occupational Therapy has Therapy for helping him build physical and helped people engage mind and body. -

Actions Synthétiques France Heures De Négociation : 9:00 - 17:30 (CET) Frais Et Commissions : 0.1% Du Montant De La Transaction, Min
Actions Synthétiques France Heures de négociation : 9:00 - 17:30 (CET) Frais et Commissions : 0.1% du montant de la transaction, min. 8 EUR (Marge sur commission: 70% - 99.9%). Symbole Instrument dont le prix est basé sur Nombre d'actions par lot Taille minimale d'un ordre en lots Vente à découvert Taux d'emprunt de titre (%) AC.FR Accor SA CFD 1 1 OUI -3 ACA.FR Credit Agricole SA CFD 1 1 OUI -3 ADP.FR Aeroports de Paris CFD 1 1 OUI -3 AF.FR Air France-KLM CFD 1 1 OUI -3 AI.FR Air Liquide SA CFD 1 1 OUI -3 AIR.FR Airbus Group NV CFD 1 1 NON - AKE.FR Arkema SA CFD 1 1 OUI -3 ALO.FR Alstom SA CFD 1 1 OUI -3 ALT.FR Altran Technologies SA CFD 1 1 OUI -3 ATO.FR AtoS CFD 1 1 OUI -3 BB.FR Societe BIC SA CFD 1 1 OUI -3 BIM.FR BioMerieux CFD 1 1 OUI -3 BN.FR Danone CFD 1 1 OUI -3 BNP.FR BNP Paribas CFD 1 1 OUI -3 BOL.FR Bollore SA CFD 1 1 OUI -3 BVI.FR Bureau Veritas SA CFD 1 1 OUI -3 CA.FR Carrefour SA CFD 1 1 OUI -3 CAP.FR Cap Gemini SA CFD 1 1 OUI -3 CGG.FR CGG SA CFD 1 1 NON - CNP.FR CNP Assurances CFD 1 1 OUI -3 CO.FR Casino Guichard Perrachon SA CFD 1 1 OUI -3 COFA.FR Coface SA CFD 1 1 OUI -4,5 CS.FR AXA SA CFD 1 1 OUI -3 DEC.FR JCDecaux SA CFD 1 1 OUI -3 DG.FR Vinci SA CFD 1 1 OUI -3 DSY.FR Dassault Systemes CFD 1 1 OUI -3 EDEN.FR Edenred CFD 1 1 OUI -3 EDF.FR EDF SA CFD 1 1 OUI -3 EI.FR Essilor International SA CFD 1 1 OUI -3 ELE.FR Euler Hermes Group CFD 1 1 OUI -4,5 EN.FR Bouygues SA CFD 1 1 OUI -3 ENGI.FR ENGIE CFD 1 1 OUI -3 ENX.FR Euronext NV CFD 1 1 OUI -3 EO.FR Faurecia CFD 1 1 OUI -3 ERA.FR Eramet CFD 1 1 OUI -5 ERF.FR Eurofins -

Mersen Urd 2020
URD 2020 Universal Registration Document MERSEN Universal Registration Document page 1 Group Profile 3 2 Corporate governance report 17 3 Management Report 75 4 Social responsibility and sustainable development 101 5 Information about the share capital and share ownership 147 6 Consolidated financial statements 165 7 Parent company financial statements 225 8 Additional information & glossaries 253 This document is a free translation into English for convenience purposes only of the French URD fi led with the Autorité des Marchés Financiers on March 15, 2021. URD 2020 | MERSEN 1 2 MERSEN | URD 2020 1 GROUP PROFILE 2020: AN UNPRECEDENTED YEAR 5 2020 KEY FIGURES 6 GROUP B USINESS M ODEL 8 VISION, MISSION AND VALUES 10 URD 2020 | MERSEN 3 GROUP PROFILE 1 4 MERSEN | URD 2020 GROUP PROFILE 2020: an unprecedented year 1 2020: AN UNPRECEDENTED YEAR After several years of growth, the Covid-19 pandemic hit the The additional direct costs caused by Covid-19 (purchase of global economy hard in 2020, with many countries introducing masks, cleaning costs, exceptional transportation, etc.) were travel restrictions, lockdown measures and quarantines to slow recorded but were for the most part offset by decreased spending the spread of the pandemic. These restrictions began in January as a result of slower business activity (especially reduced travel and February in China and then reached Europe in early March expenses). and America at the end of March. All in all, the Group demonstrated its resilience and ability to adapt Although some countries eased their lockdowns after the fi rst its expenditure, delivering operating margin of 8.1% of sales. -

FALL 2018 MSK Takes a Lead Role in Interventional Oncology FSFOCAL
FOCAL SPOT FS FALL 2018 MSK Takes a Lead Role in Interventional Oncology MALLINCKRODT INSTITUTE OF RADIOLOGY // WASHINGTON UNIVERSITY // ST. LOUIS CONTENTS 10 A look at 10 MIR professors RAD who are helping lead the way for women in radiology. (From left: Geetika Khanna, MD, Pamela K. WOMEN Woodard, MD, and Farrokh Dehdashti, MD) 6 16 20 LIFE-ALTERING MYSTERIES MIR ALUMNI TREATMENT EXPLAINED WEEKEND MSK imaging chief Jack Jennings, Thirty years later, Marcus E. Raichle, Old friends, new stories and a sweet MD, provides quality-of-life improving MD, remains a central figure in the serenade from Ronald G. Evens, MD. procedures for patients with cancer. science of brain imaging. Inside MIR’s first reunion. Cover Photo: Metastatic melanoma patient Chris Plummer still works his farm every day, thanks to treatment resulting in unprecedented control of his tumors. 2 SPOT NEWS 24 ALUMNI SPOTLIGHT FOCAL SPOT MAGAZINE FALL 2018 Editor: Marie Spadoni Photography: Mickey Wynn, Daniel Drier Design: Kim Kania YI F A LOOK BACK ©2018 Mallinckrodt Institute of Radiology 26 28 mir.wustl.edu FOCAL SPOT MAGAZINE // 1 SPOT NEWS David H. Ballard, MD, a TOP-TIER participant, uses 3D printing in his translational imaging work. Training the Next Generation of Imaging Scientists by Kristin Rattini For young scientists eager to make their way to the forefront in clinical translational imaging research and bringing of translational research and precision imaging, Mallinckrodt innovation to the practice of medicine. The interdisciplinary Institute of Radiology (MIR) offers a clear path. A leader in grant provides two training slots per year in years one NIH funding, MIR is home to premier training programs and and two, and three slots in years three through five. -

Amundi Actions Pme
SEMI-ANNUAL REPORT OCTOBER 2019 AMUNDI ACTIONS PME AMUNDI’S ASSET MANAGEMENT UCITS Fund manager Amundi Asset Management Delegated fund accountant CACEIS Fund Administration France Custodian CACEIS BANK Auditors PRICEWATERHOUSECOOPERS AUDIT UCITS AMUNDI ACTIONS PME Statement of Net Assets in EUR Elements of Statement of Net Assets Semi-Annual Report Amounts* a) Eligible financial securities mentioned in paragraph 1 of section I of Article L. 214-20 of the French 661,050,287.17 Monetary and Financial Code. b) Cash at banks and liquidities 3,919,900.98 c) Other Assets held by the UCITS 72,513,792.08 d) Total of Assets held by the UCITS (lines a+b+c) 737,483,980.23 e) Liabilities -991,745.75 f) Net Asset Value (lines d+e= net asset of the UCITS) 736,492,234.48 * Amounts are signed Number of units or shares outstanding and net asset values per unit or share Number of units Net asset value per Unit Unit type Net Assets per unit outstanding share AMUNDI ACTIONS PME C C 447,179,434.24 640,726.857 697.92 AMUNDI ACTIONS PME 0 C 203,327,023.30 1,093,251.672 185.98 AMUNDI ACTIONS PME S C 85,985,776.94 123,651.972 695.38 - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - Semi-Annual Report on 10/31/19 2 UCITS AMUNDI ACTIONS PME Items of portfolio listing Total Percentage of Items of portfolio listing Percentage Net Assets * Assets ** A) Eligible financial securities and money market instruments admitted for trading 89.76 89.64 on a regulated market pursuant to Article L. -
![Reviewers [PDF]](https://docslib.b-cdn.net/cover/7014/reviewers-pdf-667014.webp)
Reviewers [PDF]
The Journal of Neuroscience, January 2013, 33(1) Acknowledgement For Reviewers 2012 The Editors depend heavily on outside reviewers in forming opinions about papers submitted to the Journal and would like to formally thank the following individuals for their help during the past year. Kjersti Aagaard Frederic Ambroggi Craig Atencio Izhar Bar-Gad Esther Aarts Céline Amiez Coleen Atkins Jose Bargas Michelle Aarts Bagrat Amirikian Lauren Atlas Steven Barger Lawrence Abbott Nurith Amitai David Attwell Cornelia Bargmann Brandon Abbs Yael Amitai Etienne Audinat Michael Barish Keiko Abe Martine Ammasari-Teule Anthony Auger Philip Barker Nobuhito Abe Katrin Amunts Vanessa Auld Neal Barmack Ted Abel Costas Anastassiou Jesús Avila Gilad Barnea Ute Abraham Beau Ances Karen Avraham Carol Barnes Wickliffe Abraham Richard Andersen Gautam Awatramani Steven Barnes Andrey Abramov Søren Andersen Edward Awh Sue Barnett Hermann Ackermann Adam Anderson Cenk Ayata Michael Barnett-Cowan David Adams Anne Anderson Anthony Azevedo Kevin Barnham Nii Addy Clare Anderson Rony Azouz Scott Barnham Arash Afraz Lucy Anderson Hiroko Baba Colin J. Barnstable Ariel Agmon Matthew Anderson Luiz Baccalá Scott Barnum Adan Aguirre Susan Anderson Stephen Baccus Ralf Baron Geoffrey Aguirre Anuska Andjelkovic Stephen A. Back Pascal Barone Ehud Ahissar Rodrigo Andrade Lars Bäckman Maureen Barr Alaa Ahmed Ole Andreassen Aldo Badiani Luis Barros James Aimone Michael Andres David Badre Andreas Bartels Cheryl Aine Michael Andresen Wolfgang Baehr David Bartés-Fas Michael Aitken Stephen Andrews Mathias Bähr Alison Barth Elias Aizenman Thomas Andrillon Bahador Bahrami Markus Barth Katerina Akassoglou Victor Anggono Richard Baines Simon Barthelme Schahram Akbarian Fabrice Ango Jaideep Bains Edward Bartlett Colin Akerman María Cecilia Angulo Wyeth Bair Timothy Bartness Huda Akil Laurent Aniksztejn Victoria Bajo-Lorenzana Marisa Bartolomei Michael Akins Lucio Annunziato David Baker Marlene Bartos Emre Aksay Daniel Ansari Harriet Baker Jason Bartz Kaat Alaerts Mark S. -

Présentation Powerpoint
Capital Markets Day December 1, 2020 1 Disclaimer & Safe Harbor • This presentation includes only summary information and does not purport to be comprehensive. Forward-looking statements, targets and estimates contained herein are for illustrative purposes only and are based on management’s current views and assumptions. Such statements involve known and unknown risks and uncertainties that may cause actual results, performance or events to differ materially from those anticipated in the summary information. Actual results may depart significantly from these targets given the occurrence of certain risks and uncertainties, notably given that a new product can appear to be promising at a preparatory stage of development or after clinical trials but never be launched on the market or be launched on the market but fail to sell notably for regulatory or competitive reasons. The Group must deal with or may have to deal with competition from generic that may result in market share losses, which could affect its current level of growth in sales or profitability. The Company expressly disclaims any obligation or undertaking to update or revise any forward-looking statements, targets or estimates contained in this presentation to reflect any change in events, conditions, assumptions or circumstances on which any such statements are based unless so required by applicable law. • All product names listed in this document are either licensed to the Ipsen Group or are registered trademarks of the Ipsen Group or its partners. • The implementation of the strategy has to be submitted to the relevant staff representation authorities in each country concerned, in compliance with the specific procedures, terms and conditions set forth by each national legislation. -

Uva-DARE (Digital Academic Repository)
UvA-DARE (Digital Academic Repository) The space inside the skull : digital representations, brain mapping and cognitive neuroscience in the decade of the brain Beaulieu, J.A. Publication date 2000 Link to publication Citation for published version (APA): Beaulieu, J. A. (2000). The space inside the skull : digital representations, brain mapping and cognitive neuroscience in the decade of the brain. General rights It is not permitted to download or to forward/distribute the text or part of it without the consent of the author(s) and/or copyright holder(s), other than for strictly personal, individual use, unless the work is under an open content license (like Creative Commons). Disclaimer/Complaints regulations If you believe that digital publication of certain material infringes any of your rights or (privacy) interests, please let the Library know, stating your reasons. In case of a legitimate complaint, the Library will make the material inaccessible and/or remove it from the website. Please Ask the Library: https://uba.uva.nl/en/contact, or a letter to: Library of the University of Amsterdam, Secretariat, Singel 425, 1012 WP Amsterdam, The Netherlands. You will be contacted as soon as possible. UvA-DARE is a service provided by the library of the University of Amsterdam (https://dare.uva.nl) Download date:30 Sep 2021 Workss Cited Ahhot.. Allison 19922 Confusion about form and function clouds launch of EC's Decade of ihe Brain. Nature. 24 September.. 359: 260. Ackerman.. Sandra 19922 The Role of the Brain in Mental Illness. !n Discovering the Brain. 46-66. Washington. DC:: National Academy Press. -

Giovanni Berlucchi
BK-SFN-NEUROSCIENCE-131211-03_Berlucchi.indd 96 16/04/14 5:21 PM Giovanni Berlucchi BORN: Pavia, Italy May 25, 1935 EDUCATION: Liceo Classico Statale Ugo Foscolo, Pavia, Maturità (1953) Medical School, University of Pavia, MD (1959) California Institute of Technology, Postdoctoral Fellowship (1964–1965) APPOINTMENTS: University of Pennsylvania (1968) University of Siena (1974) University of Pisa (1976) University of Verona (1983) HONORS AND AWARDS: Academia Europaea (1990) Accademia Nazionale dei Lincei (1992) Honorary PhD in Psychology, University of Pavia (2007) After working initially on the neurophysiology of the sleep-wake cycle, Giovanni Berlucchi did pioneering electrophysiological investigations on the corpus callosum and its functional contribution to the interhemispheric transfer of visual information and to the representation of the visual field in the cerebral cortex and the superior colliculus. He was among the first to use reaction times for analyzing hemispheric specializations and interactions in intact and split brain humans. His latest research interests include visual spatial attention and the representation of the body in the brain. BK-SFN-NEUROSCIENCE-131211-03_Berlucchi.indd 97 16/04/14 5:21 PM Giovanni Berlucchi Family and Early Years A man’s deepest roots are where he has spent the enchanted days of his childhood, usually where he was born. My deepest roots lie in the ancient Lombard city of Pavia, where I was born 78 years ago, on May 25, 1935, and in that part of the province of Pavia that lies to the south of the Po River and is called the Oltrepò Pavese. The hilly part of the Oltrepò is covered with beautiful vineyards that according to archaeological and historical evidence have been used to produce good wines for millennia.