Présentation Powerpoint
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

———— 2016 Registration Document
———— 2016 REGISTRATION DOCUMENT Including the annual financial report 01 Persons responsible 3 16 Practices of the governing bodies 91 02 Statutory Auditors 5 17 Employees 93 17.1 Employee data 93 03 Selected financial information 7 17.2 Employee share-ownership and profit-sharing 95 04 Risks 11 18 Major shareholders 97 4.1 Risks specific to the Group’s activity 11 18.1 Shareholders and their voting rights 98 4.2 Liquidity risk 14 18.2 Transactions carried out 4.3 Interest rate risk 15 during the year subject to 4.4 Exchange-rate risk 16 Article L. 621-18-2 of the French 4.5 Intangible asset risk 16 Monetary and Financial Code 100 contents 4.6 Environment risk 17 18.3 Share buybacks 101 4.7 Legal and fiscal risks 17 18.4 Market for Altran Technologies securities 102 4.8 Investment risk 17 18.5 Information on the calculation methods 4.9 Brexit risk 18 and effects of adjustments to the conditions covering the subscription or 05 Company information 19 purchase of rights and securities giving 5.1 Company background information and access to the Company’s share capital 103 development 19 18.6 Agreements entered into by the 5.2 Main investments 20 Company which would be amended or terminated upon a change of control of 06 Information about the the Company 103 Group’s businesses 21 18.7 Agreements between shareholders, of 6.1 Core activities 21 which the Company is aware and which may cause restrictions to the transfer of 6.2 The Engineering and R&D Services market 22 shares and/or the exercise of voting rights 104 6.3 Competition 24 18.8 Commitments -
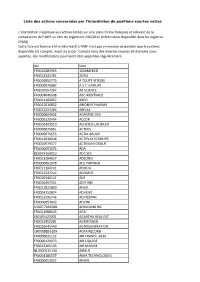
Liste Des Actions Concernées Par L'interdiction De Positions Courtes Nettes
Liste des actions concernées par l'interdiction de positions courtes nettes L’interdiction s’applique aux actions listées sur une plate-forme française et relevant de la compétence de l’AMF au titre du règlement 236/2012 (information disponible dans les registres ESMA). Cette liste est fournie à titre informatif. L'AMF n'est pas en mesure de garantir que le contenu disponible est complet, exact ou à jour. Compte tenu des diverses sources de données sous- jacentes, des modifications pourraient être apportées régulièrement. Isin Nom FR0010285965 1000MERCIS FR0013341781 2CRSI FR0010050773 A TOUTE VITESSE FR0000076887 A.S.T. GROUPE FR0010557264 AB SCIENCE FR0004040608 ABC ARBITRAGE FR0013185857 ABEO FR0012616852 ABIONYX PHARMA FR0012333284 ABIVAX FR0000064602 ACANTHE DEV. FR0000120404 ACCOR FR0010493510 ACHETER-LOUER.FR FR0000076861 ACTEOS FR0000076655 ACTIA GROUP FR0011038348 ACTIPLAY (GROUPE) FR0010979377 ACTIVIUM GROUP FR0000053076 ADA BE0974269012 ADC SIIC FR0013284627 ADEUNIS FR0000062978 ADL PARTNER FR0011184241 ADOCIA FR0013247244 ADOMOS FR0010340141 ADP FR0010457531 ADTHINK FR0012821890 ADUX FR0004152874 ADVENIS FR0013296746 ADVICENNE FR0000053043 ADVINI US00774B2088 AERKOMM INC FR0011908045 AG3I ES0105422002 AGARTHA REAL EST FR0013452281 AGRIPOWER FR0010641449 AGROGENERATION CH0008853209 AGTA RECORD FR0000031122 AIR FRANCE -KLM FR0000120073 AIR LIQUIDE FR0013285103 AIR MARINE NL0000235190 AIRBUS FR0004180537 AKKA TECHNOLOGIES FR0000053027 AKWEL FR0000060402 ALBIOMA FR0013258662 ALD FR0000054652 ALES GROUPE FR0000053324 ALPES (COMPAGNIE) -

Actions Synthétiques France Heures De Négociation : 9:00 - 17:30 (CET) Frais Et Commissions : 0.1% Du Montant De La Transaction, Min
Actions Synthétiques France Heures de négociation : 9:00 - 17:30 (CET) Frais et Commissions : 0.1% du montant de la transaction, min. 8 EUR (Marge sur commission: 70% - 99.9%). Symbole Instrument dont le prix est basé sur Nombre d'actions par lot Taille minimale d'un ordre en lots Vente à découvert Taux d'emprunt de titre (%) AC.FR Accor SA CFD 1 1 OUI -3 ACA.FR Credit Agricole SA CFD 1 1 OUI -3 ADP.FR Aeroports de Paris CFD 1 1 OUI -3 AF.FR Air France-KLM CFD 1 1 OUI -3 AI.FR Air Liquide SA CFD 1 1 OUI -3 AIR.FR Airbus Group NV CFD 1 1 NON - AKE.FR Arkema SA CFD 1 1 OUI -3 ALO.FR Alstom SA CFD 1 1 OUI -3 ALT.FR Altran Technologies SA CFD 1 1 OUI -3 ATO.FR AtoS CFD 1 1 OUI -3 BB.FR Societe BIC SA CFD 1 1 OUI -3 BIM.FR BioMerieux CFD 1 1 OUI -3 BN.FR Danone CFD 1 1 OUI -3 BNP.FR BNP Paribas CFD 1 1 OUI -3 BOL.FR Bollore SA CFD 1 1 OUI -3 BVI.FR Bureau Veritas SA CFD 1 1 OUI -3 CA.FR Carrefour SA CFD 1 1 OUI -3 CAP.FR Cap Gemini SA CFD 1 1 OUI -3 CGG.FR CGG SA CFD 1 1 NON - CNP.FR CNP Assurances CFD 1 1 OUI -3 CO.FR Casino Guichard Perrachon SA CFD 1 1 OUI -3 COFA.FR Coface SA CFD 1 1 OUI -4,5 CS.FR AXA SA CFD 1 1 OUI -3 DEC.FR JCDecaux SA CFD 1 1 OUI -3 DG.FR Vinci SA CFD 1 1 OUI -3 DSY.FR Dassault Systemes CFD 1 1 OUI -3 EDEN.FR Edenred CFD 1 1 OUI -3 EDF.FR EDF SA CFD 1 1 OUI -3 EI.FR Essilor International SA CFD 1 1 OUI -3 ELE.FR Euler Hermes Group CFD 1 1 OUI -4,5 EN.FR Bouygues SA CFD 1 1 OUI -3 ENGI.FR ENGIE CFD 1 1 OUI -3 ENX.FR Euronext NV CFD 1 1 OUI -3 EO.FR Faurecia CFD 1 1 OUI -3 ERA.FR Eramet CFD 1 1 OUI -5 ERF.FR Eurofins -

Mersen Urd 2020
URD 2020 Universal Registration Document MERSEN Universal Registration Document page 1 Group Profile 3 2 Corporate governance report 17 3 Management Report 75 4 Social responsibility and sustainable development 101 5 Information about the share capital and share ownership 147 6 Consolidated financial statements 165 7 Parent company financial statements 225 8 Additional information & glossaries 253 This document is a free translation into English for convenience purposes only of the French URD fi led with the Autorité des Marchés Financiers on March 15, 2021. URD 2020 | MERSEN 1 2 MERSEN | URD 2020 1 GROUP PROFILE 2020: AN UNPRECEDENTED YEAR 5 2020 KEY FIGURES 6 GROUP B USINESS M ODEL 8 VISION, MISSION AND VALUES 10 URD 2020 | MERSEN 3 GROUP PROFILE 1 4 MERSEN | URD 2020 GROUP PROFILE 2020: an unprecedented year 1 2020: AN UNPRECEDENTED YEAR After several years of growth, the Covid-19 pandemic hit the The additional direct costs caused by Covid-19 (purchase of global economy hard in 2020, with many countries introducing masks, cleaning costs, exceptional transportation, etc.) were travel restrictions, lockdown measures and quarantines to slow recorded but were for the most part offset by decreased spending the spread of the pandemic. These restrictions began in January as a result of slower business activity (especially reduced travel and February in China and then reached Europe in early March expenses). and America at the end of March. All in all, the Group demonstrated its resilience and ability to adapt Although some countries eased their lockdowns after the fi rst its expenditure, delivering operating margin of 8.1% of sales. -

Amundi Actions Pme
SEMI-ANNUAL REPORT OCTOBER 2019 AMUNDI ACTIONS PME AMUNDI’S ASSET MANAGEMENT UCITS Fund manager Amundi Asset Management Delegated fund accountant CACEIS Fund Administration France Custodian CACEIS BANK Auditors PRICEWATERHOUSECOOPERS AUDIT UCITS AMUNDI ACTIONS PME Statement of Net Assets in EUR Elements of Statement of Net Assets Semi-Annual Report Amounts* a) Eligible financial securities mentioned in paragraph 1 of section I of Article L. 214-20 of the French 661,050,287.17 Monetary and Financial Code. b) Cash at banks and liquidities 3,919,900.98 c) Other Assets held by the UCITS 72,513,792.08 d) Total of Assets held by the UCITS (lines a+b+c) 737,483,980.23 e) Liabilities -991,745.75 f) Net Asset Value (lines d+e= net asset of the UCITS) 736,492,234.48 * Amounts are signed Number of units or shares outstanding and net asset values per unit or share Number of units Net asset value per Unit Unit type Net Assets per unit outstanding share AMUNDI ACTIONS PME C C 447,179,434.24 640,726.857 697.92 AMUNDI ACTIONS PME 0 C 203,327,023.30 1,093,251.672 185.98 AMUNDI ACTIONS PME S C 85,985,776.94 123,651.972 695.38 - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - Semi-Annual Report on 10/31/19 2 UCITS AMUNDI ACTIONS PME Items of portfolio listing Total Percentage of Items of portfolio listing Percentage Net Assets * Assets ** A) Eligible financial securities and money market instruments admitted for trading 89.76 89.64 on a regulated market pursuant to Article L. -

2018 Survey Report Table of Contents Executive Summary
Antitrust Writing Awards 2018 ANTITRUST PROFESSIONAL PUBLICATIONS NEWSLETTERS & CLIENT ALERTS 2018201 SURVEYSURVEY REPORTREPORT POOL & METHODOLOGY The Survey was sent from November 14, 2017 to December 4, 2017 to 6,500 in-house counsels. The counsels interviewed cover more than 15 industries. Among these counsels, 25 % are General Counsels and 75% Antitrust Counsels. Individual answers are kept confidential; only aggregated data are provided herein. Survey Coverage per Geographical Area Survey Coverage per Industry 10 % 13 11 10 % 10 9 8 8 8 Europe 7 America 6 5 5 Asia 40 % 4 3 3 10 % Africa Oceania Energy Media Luxury Automobile Transports Other Industry Entertainment Other Sevices Other Industry Agricultural Products Information Technology 30 % Financial Services/Insurance Pharmaceuticals/ChemicalTelecommunications/Postal Industry Services Survey Coverage: Represented Corporations (excerpt) Aerospace/Defense Airbus, Boeing, Dassault, EADS, Safran, Snecma, Thales... Agriculture/Food Products AB-InBev, Coca-Cola, Bacardi, Kraft, Nestle, Panzani, Pepsico, Saint Louis Sucre... Automobile Ford, General Motors, Nissan, PSA, Renault, Toyota, Volkswagen, Volvo... Energy American Electric Power, BP, E-On, EDF, Exxon, Framatome, GDF Suez, IFP, Powernext, RTE, Shell, Suez Tractebel, Total... Financial Services/Insurance ABN Amro, AGF, American Express, AMF, Axa, Bank of America, Banksys, Banque postale, BNP Paribas, Calyon Bank, Cetelem, CDC, CIC, Cinven, Citigroup, Clinvest, Coface, Credit mutuel, Eurazeo, Euronext, Exane, FBF, Fortis, Groupama, ING, IXIS, JP Morgan, Lazard, Mastercard, Rothschild, Scor, Société Générale, Swift, Thomas Cook, UBS, Weinberg Capital, Wendel, Winterthur, World Bank... Entertainment 21st Century Fox, Clear Channel, Time Warner, Viacom, Walt Disney, Warner Music... Information Technology Amazon, Apple, Ericsson, Google, Hewlett-Packard, IBM, Iliad, LD Com, Microsoft, Nexans, Oracle, Qualcomm, Rim, Samsung, Sony, Spot, Sun Microsystems, Symantec.. -
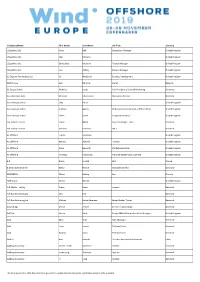
Company Name First Name Last Name Job Title Country
Company Name First Name Last Name Job Title Country 1StopWind Ltd Arran Bell Operations Manager United Kingdom 1StopWind Ltd. Alan Mckerns United Kingdom 1StopWind Ltd. Bernadette McAulay Finance Manager United Kingdom 1StopWind Ltd. Joel Telling General Manager United Kingdom 23 Degrees Renewables Ltd Ed Woodrow Business Development United Kingdom 24SEA bvba Gert De Sitter Owner Belgium 3S Europe GmbH Matthias Lamp Vice President of Sales & Marketing Germany 3sun Denmark ApS Christian Christensen Operations Director Denmark 3sun Group Limited Jody Potter United Kingdom 3sun Group Limited Graham Hacon VP Business Development, Offshore Wind United Kingdom 3sun Group Limited Sherri Smith Company Secretary United Kingdom 3W Industri Service Simon Øland Project manager - sales Denmark 3W Industri Service Kenneth Pedersen IWI-S Denmark 4C Offshore Lauren Anderson United Kingdom 4C Offshore Richard Aukland Director United Kingdom 4C Offshore Rosie Haworth Market Researcher United Kingdom 4C Offshore Vincenzo Poidomani Principal Geotechnical Engineer United Kingdom 8.2 Bruno ALLAIN CEO France 8.2 Monitoring GmbH Bernd Höring Managing director Germany 920338402 Ellinor Meling Ceo Norway A&P Group Emma Harrick United Kingdom A.P. Møller Holding Simon Ibsen Investor Denmark A/S Dan-Bunkering Ltd. Jens Kirk Denmark A/S Dan-Bunkering Ltd. Michael Brunø-Sørensen Senior Bunker Trader Denmark A1wind Aps Martin Jensen Director / A1wind Aps Denmark AAF Ltd Steven Brett Europe MFAS Aftermarket Sales Manager United Kingdom AAG Allan Tarp Sales Manager Denmark -

Le Patient Est Dans Notre Adn
LE PATIENT EST DANS NOTRE ADN DOCUMENT DE RÉFÉRENCE 2014 SOMMAIRE REMARQUES GÉNÉRALES 2 2.1.6 Rapport des Commissaires aux comptes sur les comptes consolidés 167 CALENDRIER INDICATIF 2.2 Comptes sociaux 2014 169 DE LA COMMUNICATION FINANCIÈRE 3 2.2.1 Documents de synthèse 169 INTRODUCTION : PRÉSENTATION GÉNÉRALE 4 2.2.2 Annexe aux comptes annuels 172 2.2.3 Rapport des Commissaires aux comptes 1 PRÉSENTATION D’IPSEN ET DE SON ACTIVITÉ 5 sur les comptes annuels 187 1.1 Présentation générale du Groupe et de sa stratégie 6 2.2.4 Informations relatives à l’activité de Ipsen 188 1.1.1 Historique, évolution et stratégie du Groupe 6 1.1.2 Facteurs de Risques 10 3 GOUVERNEMENT D’ENTREPRISE 1.1.3 Chiffres clés 21 ET INFORMATIONS JURIDIQUES 191 1.2 Activité du Groupe au cours de l’exercice 3.1 Gouvernement d’entreprise 192 et structure juridique 27 3.1.1 Présentation du Conseil d’administration 1.2.1 Présentation des produits du Groupe 27 et de la Direction générale 192 1.2.2 Activités en matière de Recherche et Développement 42 3.1.2 Rapports du Président et des Commissaires 1.2.3 Principaux marchés 49 aux comptes 209 1.2.4 Réglementation 50 3.1.3 Montant global des rémunérations 1.2.5 Effort de productivité 53 des mandataires sociaux 224 1.2.6 Analyse du résultat 54 3.1.4 Conventions conclues par le Groupe avec 1.2.7 Trésorerie et capitaux 66 ses dirigeants et principaux actionnaires 1.2.8 Structure juridique du Groupe 68 et rapport des Commissaires aux comptes 232 1.3 Informations sociales et environnementales 3.2 Renseignements concernant la -

2007 150 30 60%
Leading consulting firm in the energy transition and in climate change adaptation Founded in 2007 by two renowned experts in energy and climate change issues, Jean-Marc Jancovici and Alain Grandjean, Carbone 4 is the first consulting firm specialized in low-carbon strategy and climate change adaptation. During the course of the 800 missions carried out in France and globally, Carbone 4 has developed multiple innovative analysis methods as well as close to ten sectorial methods for carbon footprint calculations. The work of our firm has inspired the strategy of many major groups and local authorities. OUR APPROACH : FROM ANALYSIS TO AN ACTION PLAN • UNDERSTAND the stakes involved in transitioning your business and identify low-carbon projects with high added value. • ANTICIPATE the transition of our climate and economic system. • ACT ! seize the opportunities and reduce associated risks. • PROMOTE your actions, progress and carbon strategy. OUR MAIN INTERVENTION SECTORS OUR SERVICES • Low-carbon strategies Buildings Energy • Forward-looking scenarios and market studies • Carbon footprint and reporting practices • Impact assessments and competitive Finance Transport advantage reports • Climate change risks and adaptation analyses Industry Media & services • Training, seminars and conferences • Climate impact assessments of portfolios Public sector OUR KEY FIGURES 800 2007 30 Missions carried out in Creation of Carbone 4 Team members France and globally 150 60% Client references Of the CAC40 are our clients SETTING UP A BUSINESS STRATEGY INLINE WITH A 2° SCENARIO IMPLEMENTATION OF A 2° STRATEGY Carbone 4 is an expert in climate change issues, as well as in the operational levers that exist to engage in the energy transition today. -

France Alpha 7.1% Beta 0.6 Download
CIM algo Draft version R30 France Calculation Date: 2020-01-20 The CIMalgo R30 France is Cimalgo's Robust investment model applied to France. The Robust model is an algorithmic portfolio construction procedure which seeks to provide strong risk-adjusted performance and low beta in any market worldwide by aiming at high-liquidity stocks and combining high diversification with propriatary portfolio optimization techniques. The performance is achieved through optimization for low volatility and limited drawdowns. Combined with a simple equal-weighting rule ex- ploiting long-term mean reversal properties of equity prices results in stable long-term risk-adjusted performance independent of both domestic and global market business cycles. All performance below is based on total return. Backtest Performance Model Characteristics Backtested performance is compared to MSCI France Gross Total Return USD Index. Region France 2000−01−10 / 2020−01−20 Universe All Trading Constituents 30 600 R30 MSCI Weighting Equal 500 Rebalancing Quarterly 400 Currency USD Liquidity Requirement Min 0.2M USD ADTV 300 Annual Performance (%) 200 Year Return % Volatility % Sharpe Ratio 100 R30 MSCI R30 MSCI R30 MSCI 2000 21.1 0.4 21.6 24.5 1.0 0.0 Jan 10 Jan 01 Jan 01 Jan 02 Jan 01 Jan 01 Jan 02 Jan 01 Jan 01 Jan 01 Jan 01 2000 2002 2004 2006 2008 2010 2012 2014 2016 2018 2020 2001 -15.6 -22.1 17.2 25.0 -0.9 -0.9 2002 5.1 -20.8 13.9 32.0 0.4 -0.7 2003 41.6 41.0 10.2 22.2 4.1 1.9 Drawdown Comparison (%) 2004 36.8 19.2 12.0 15.0 3.1 1.3 2005 13.7 10.6 10.9 12.4 1.3 0.9 Drawdown is compared to MSCI France Gross Total Return USD Index. -

Corporate Governance
6 CORPORATE GOVERNANCE 6.3.3 Operating procedures and work 6.1 SAFRAN’S CORPORATE of the Board of Directors 357 GOVERNANCE STRUCTURE 318 6.3.4 Committees of the Board of Directors 361 Corporate governance reference framework 318 6.3.5 Self-assessment by the Board 6.1.1 Board of Directors – Separation of the roles of its operating procedures 367 of Chairman of the Board of Directors and Chief Executive Officer 318 6.4 APPLICATION 6.1.2 Powers and responsibilities of the Chairman OF THE AFEP–MEDEF of the Board of Directors 318 CORPORATE GOVERNANCE CODE 368 6.1.3 Powers and responsibilities of the Chief Executive Officer 319 6.5 DIRECTORS’ INTERESTS IN 6.1.4 Powers and responsibilities THE COMPANY’S SHARE CAPITAL 369 of the Board of Directors 319 6.5.1 Compulsory shareholdings 369 6.2 MEMBERSHIP STRUCTURE 6.5.2 Code of Ethics 369 OF THE BOARD OF DIRECTORS 321 6.5.3 Transactions in the Company’s shares carried out by corporate officers 6.2.1 Summary table of information and senior managers 370 about Directors (at the filing date of this Universal Registration Document) 322 6.6 COMPENSATION POLICY 6.2.2 Directors’ profiles (at the filing date AND COMPENSATION PACKAGES of this Universal Registration Document) 326 FOR CORPORATE OFFICERS 371 6.2.3 Other information about the Board 6.6.1 Compensation policy of Directors’ membership structure 346 for corporate officers – 2021 371 6.2.4 Independence and diversity 6.6.2 Compensation and benefits of the Chairman of the Board of Directors 349 and the Chief Executive Officer for 2020 6.2.5 Additional -

Press Release AFEP LARGE MEMBER COMPANIES INVEST in CLIMATE
Press release Paris – July 7, 2021 AFEP LARGE MEMBER COMPANIES INVEST IN CLIMATE ACTION WITH CONCRETE PROJECTS Ahead of the COP 26, AFEP launches Ambition 4 Climate, a platform to present on- going low-carbon projects and dialogue with stakeholders While green transition is a major concern and a driver for action, the members of the French Association of Large Companies (AFEP) are committed to tackle the climate emergency. As part of their climate strategy, they are developing a diversity of replicable low-carbon projects that provide concrete solutions to contribute to the climate neutrality challenge. AFEP launches the platform Ambition 4 Climate, which illustrates large companies’ mobilisation to reduce their greenhouse gas (GHG) emissions throughout their value chains with specific examples in a wide range of economic sectors. Being ambitious for the climate means acting! Ambition 4 Climate is a dedicated Internet platform that brings together concrete initiatives taken by AFEP member companies to fight climate change. While investing in various know-how, innovations and technologies, large French companies are implementing operational low-carbon solutions throughout their value chains. Beyond their own action, they are triggering a leverage effect on their ecosystems. Ambition 4 Climate brings together a variety of actions currently being implemented by companies from different sectors to reduce their GHG emissions and those of their suppliers, customers, and partners. These projects, presented in the form of factual data and figures, result from recent investment decisions. They lead to significant changes in production processes, products, and everyday business life. For each project, the carbon impact is assessed according to a detailed methodology.