Giant Cell Arteritis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Fundus Signs in Temporal Arteritis
Br J Ophthalmol: first published as 10.1136/bjo.62.9.591 on 1 September 1978. Downloaded from British Journal of Ophthalmology, 1978, 62, 591-594 Fundus signs in temporal arteritis D. McLEOD, E. 0. OJI, E. M. KOHNER, AND J. MARSHALL From Moorfields Eye Hospital and Institute of Ophthalmology, London SUMMARY A patient with temporal arteritis developed a variety of ischaemic lesions in the eyes. Infarction of the inner retina and optic nerve head was delineated on presentation by white swelling in the retinal nerve fibre layer. The role of interrupted axoplasmic transport in the production of this sign is discussed. Outer retinal infarction was also noted on presentation and subsequently gave rise to striking pigmented scars. Temporal arteritis often presents with visual loss, the central venous tributaries were of normal and necropsy examination in such cases shows wide- calibre and colour. No abnormality of the inner spread disease of the ophthalmic artery and the retina was noted in the territory of supply of the extraocular course of its ciliary and retinal branches central retinal artery. (Henkind et al., 1970). The medial and lateral At first sight the left eye showed a similar ophthal- posterior ciliary arteries supply the optic nerve head, moscopic picture, with pale swelling of the nasal the outer retina, and, in 20 to 50% of individuals, part of the optic disc and a row of fluffy white by copyright. a variable area of inner retina contiguous with the cotton-wool spots crossing the papillomacular optic disc (Hayreh, 1969); the central retinal artery bundle (Fig. 2). -

Microsurgical Anatomy of the Central Retinal Artery
Original Article Microsurgical Anatomy of the Central Retinal Artery Matias Baldoncini1,2, Alvaro Campero2,3, Gabriel Moran1, Maximiliano Avendan˜ o1, Pablo Hinojosa-Martı´nez1, Marcela Cimmino1, Pablo Buosi1, Valeria Forlizzi1, Joaquı´n Chuang1, Brian Gargurevich1 - BACKGROUND: The central retinal artery (CRA) has INTRODUCTION been described as one of the first branches of the he central retinal artery (CRA) is described as one of the ophthalmic artery.It arises medial to the ciliary ganglion first branches of the ophthalmic artery. It arises medial to and after a sinuous path within the orbital cavity it pene- T the ciliary ganglion, and after a sinuous path inside the trates the lower surface of the dura mater that covers the orbital cavity, penetrates the lower surface of the dura mater that optic nerve, approximately 1 cm behind the eyeball. How- covers the optic nerve, approximately 1 cm behind the eyeball. ever, the numerous anatomic descriptions that were made After a short journey inside this meninge, it crosses the cranial of the CRA have been insufficient or unclear in relation to nerve to be located in its center and travels until it reaches the optical papilla where it divides into several branches. During this certain characteristics that are analyzed in the present entire journey, the CRA does not present any anastomoses, study. considering it as a terminal branch.1-4 However, the descriptions that many investigators make about - METHODS: An electronic literature search was made in some of thesecharacteristics of the CRA differ with the classic the PubMed database and a cadaver dissection was per- disposition] (Tables 1e12).5-40 The numerous anatomic de- formed on 11 orbits fixed in formaldehyde. -

THE CENTRAL ARTERY of the RETINA*T H
Br J Ophthalmol: first published as 10.1136/bjo.44.5.280 on 1 May 1960. Downloaded from Brit. J. Ophthal. (1960) 44, 280. THE CENTRAL ARTERY OF THE RETINA*t H. A STUDY OF ITS DISTRIBUHTION AND ANASTOMOSES BY SOHAN SINGH AND RAMJI DASS Department ofAnatomy, Government Medical College, Patiala, India THERE is little unanimity regarding the distribution and anastomoses of the central artery of the retina. According to Frangois and Neetens (1954, 1956) and Fran9ois, Neetens, and Collette (1955), the central artery of the retina is completely devoid of branches, while according to Wolff (1939) and Behr (1935) it is free from branches only in its intraneural course. Magitot (1908), Quain (1909), Beauvieux and Ristitch (1924), Wolff (1940), Bignell (1952), Wybar (1956), and Steele and Blunt (1956) on the other hand demon- strated branches from all parts of its course. Most investigators agree that this artery contributes to the blood supply of the optic nerve, but Francois and others (1954, 1955, 1956) affirm that this is not the case. The presence of a central artery of the optic nerve described by Behr (1935), Wolff (1939, 1954), Francois and others (1954, 1955, 1956), and Wybar (1956), is denied by Beauvieux and Ristitch (1924) and Steele and Blunt (1956). Beauvieux and Ristitch (1924), Kershner (1943), Frangois and others http://bjo.bmj.com/ (1954, 1955, 1956), and Steele and Blunt (1956) say there are no anastomoses between the central retinal and other arteries, but Vail (1948), Wybar (1956), and several other authors have observed and described them. The lamina cribrosa is the only site at which these anastomoses have been studied in any detail, and in this case too, contradictory views have been expressed. -

ANCA--Associated Small-Vessel Vasculitis
ANCA–Associated Small-Vessel Vasculitis ISHAK A. MANSI, M.D., PH.D., ADRIANA OPRAN, M.D., and FRED ROSNER, M.D. Mount Sinai Services at Queens Hospital Center, Jamaica, New York and the Mount Sinai School of Medicine, New York, New York Antineutrophil cytoplasmic antibodies (ANCA)–associated vasculitis is the most common primary sys- temic small-vessel vasculitis to occur in adults. Although the etiology is not always known, the inci- dence of vasculitis is increasing, and the diagnosis and management of patients may be challenging because of its relative infrequency, changing nomenclature, and variability of clinical expression. Advances in clinical management have been achieved during the past few years, and many ongoing studies are pending. Vasculitis may affect the large, medium, or small blood vessels. Small-vessel vas- culitis may be further classified as ANCA-associated or non-ANCA–associated vasculitis. ANCA–asso- ciated small-vessel vasculitis includes microscopic polyangiitis, Wegener’s granulomatosis, Churg- Strauss syndrome, and drug-induced vasculitis. Better definition criteria and advancement in the technologies make these diagnoses increasingly common. Features that may aid in defining the spe- cific type of vasculitic disorder include the type of organ involvement, presence and type of ANCA (myeloperoxidase–ANCA or proteinase 3–ANCA), presence of serum cryoglobulins, and the presence of evidence for granulomatous inflammation. Family physicians should be familiar with this group of vasculitic disorders to reach a prompt diagnosis and initiate treatment to prevent end-organ dam- age. Treatment usually includes corticosteroid and immunosuppressive therapy. (Am Fam Physician 2002;65:1615-20. Copyright© 2002 American Academy of Family Physicians.) asculitis is a process caused These antibodies can be detected with indi- by inflammation of blood rect immunofluorescence microscopy. -

A Review of Primary Vasculitis Mimickers Based on the Chapel Hill Consensus Classification
Hindawi International Journal of Rheumatology Volume 2020, Article ID 8392542, 11 pages https://doi.org/10.1155/2020/8392542 Review Article A Review of Primary Vasculitis Mimickers Based on the Chapel Hill Consensus Classification Farah Zarka ,1 Charles Veillette ,1 and Jean-Paul Makhzoum 2 1Hôpital du Sacré-Cœur de Montreal, University of Montreal, Canada 2Vasculitis Clinic, Department of Internal Medicine, Hôpital du Sacré-Coeur de Montreal, University of Montreal, Canada Correspondence should be addressed to Jean-Paul Makhzoum; [email protected] Received 10 July 2019; Accepted 7 January 2020; Published 18 February 2020 Academic Editor: Charles J. Malemud Copyright © 2020 Farah Zarka et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Primary systemic vasculitides are rare diseases that may manifest similarly to more commonly encountered conditions. Depending on the size of the vessel affected (large vessel, medium vessel, or small vessel), different vasculitis mimics must be considered. Establishing the right diagnosis of a vasculitis mimic will prevent unnecessary immunosuppressive therapy. 1. Introduction 2. Large-Vessel Vasculitis Mimics Vasculitides are rare heterogenous diseases that affect vessel Large-vessel vasculitis (LVV) is an inflammatory vascu- walls as the main site of inflammation. Organs affected vary lopathy affecting large arteries; giant cell arteritis (GCA) depending on the type and size of blood vessels involved and Takayasu’s arteritis (TAK) are the two main docu- [1]. Autoimmune vasculitis can be primary (idiopathic) or mented variants, each with their own characteristic fea- secondary to an underlying disease. -
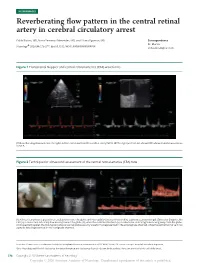
Reverberating Flow Pattern in the Central Retinal Artery in Cerebral
NEUROIMAGES Reverberating flow pattern in the central retinal artery in cerebral circulatory arrest Pablo Blanco, MD, Mar´ıa Fernanda Men´endez, MD, and Liliana Figueroa, MD Correspondence Dr. Blanco Neurology 2020;94:276-277. doi:10.1212/WNL.0000000000008918 ® [email protected] Figure 1 Transcranial Doppler and central retinal arteries (CRA) waveforms (A) Reverberating flow pattern in the right (and left, not shown) middle cerebral artery (MCA). (B) The right (and left, not shown) CRA showed similar waveforms to MCA. Figure 2 Technique for ultrasound assessment of the central retinal arteries (CRA) flow (A) A linear transducer is placed in an axial position over the globe, with the eyelids closed and covered by a generous amount of gel. (B) In color Doppler, the CRA (a) is coded red, indicating flow moving toward the globe (G), while the central retinal vein (v) is coded blue, indicating flow moving away from the globe. (C) In spectral Doppler, the CRA typically shows low resistance velocity waveforms (represented in the anterograde channel), while the central retinal vein has a phasic flow (represented in the retrograde channel). From the “Centro Unico´ Coordinador de Ablacion´ e Implante Provincia de Buenos Aires (CUCAIBA)” Team, “Dr. Emilio Ferreyra” Hospital, Necochea, Argentina. Go to Neurology.org/N for full disclosures. Funding information and disclosures deemed relevant by the authors, if any, are provided at the end of the article. 276 Copyright © 2020 American Academy of Neurology Copyright © 2020 American Academy of Neurology. Unauthorized reproduction of this article is prohibited. A 49-year-old woman developed signs of brain death after when obtaining flow signals through the cranial bone is not a severe traumatic brain injury. -

The Ophthalmic Artery Ii
Brit. J. Ophthal. (1962) 46, 165. THE OPHTHALMIC ARTERY II. INTRA-ORBITAL COURSE* BY SOHAN SINGH HAYREHt AND RAMJI DASS Government Medical College, Patiala, India Material THIS study was carried out in 61 human orbits obtained from 38 dissection- room cadavers. In 23 cadavers both the orbits were examined, and in the remaining fifteen only one side was studied. With the exception of three cadavers of children aged 4, 11, and 12 years, the specimens were from old persons. Method Neoprene latex was injected in situ, either through the internal carotid artery or through the most proximal part of the ophthalmic artery, after opening the skull and removing the brain. The artery was first irrigated with water. After injection the part was covered with cotton wool soaked in 10 per cent. formalin for from 24 to 48 hours to coagulate the latex. The roof of the orbit was then opened and the ophthalmic artery was carefully studied within the orbit. Observations COURSE For descriptive purposes the intra-orbital course of the ophthalmic artery has been divided into three parts (Singh and Dass, 1960). (1) The first part extends from the point of entrance of the ophthalmic artery into the orbit to the point where the artery bends to become the second part. This part usually runs along the infero-lateral aspect of the optic nerve. (2) The second part crosses over or under the optic nerve running in a medial direction from the infero-lateral to the supero-medial aspect of the nerve. (3) The thirdpart extends from the point at which the second part bends at the supero-medial aspect of the optic nerve to its termination. -

A Review of Central Retinal Artery Occlusion: Clinical Presentation And
Eye (2013) 27, 688–697 & 2013 Macmillan Publishers Limited All rights reserved 0950-222X/13 www.nature.com/eye 1 2 1 2 REVIEW A review of central DD Varma , S Cugati , AW Lee and CS Chen retinal artery occlusion: clinical presentation and management Abstract Central retinal artery occlusion (CRAO) is an that in turn place an individual at risk of future ophthalmic emergency and the ocular ana- cerebral stroke and ischaemic heart disease. logue of cerebral stroke. Best evidence reflects Although analogous to a cerebral stroke, there that over three-quarters of patients suffer is currently no guideline-endorsed evidence for profound acute visual loss with a visual acuity treatment. Current options for therapy include of 20/400 or worse. This results in a reduced the so-called ‘standard’ therapies, such as functional capacity and quality of life. There is sublingual isosorbide dinitrate, systemic also an increased risk of subsequent cerebral pentoxifylline or inhalation of a carbogen, stroke and ischaemic heart disease. There are hyperbaric oxygen, ocular massage, globe no current guideline-endorsed therapies, compression, intravenous acetazolamide and although the use of tissue plasminogen acti- mannitol, anterior chamber paracentesis, and vator (tPA) has been investigated in two methylprednisolone. None of these therapies randomized controlled trials. This review will has been shown to be better than placebo.5 describe the pathophysiology, epidemiology, There has been recent interest in the use of and clinical features of CRAO, and discuss tissue plasminogen activator (tPA) with two current and future treatments, including the recent randomized controlled trials on the 1Flinders Comprehensive use of tPA in further clinical trials. -

Rheumatology 2 Objectives
1 RHEUMATOLOGY 2 OBJECTIVES Know and understand: • How the clinical presentations of rheumatologic diseases can vary • Components of a thorough physical examination for investigating rheumatoid complaints • How to differentiate between different rheumatologic diseases • Evidence-based management of rheumatologic diseases 3 TOPICS COVERED • Osteoarthritis • Rheumatoid Arthritis • Gout • Calcium Pyrophosphate Deposition Disease • Polymyalgia Rheumatica • Giant Cell Arteritis (Temporal Arteritis) • Systemic Lupus Erythematosus • Sjögren Syndrome • Polymyositis and Dermatomyositis • Fibromyalgia 4 OSTEOARTHRITIS (OA): OVERVIEW • Principal cause of knee, hip, and back pain in older adults, and most common source of chronic pain • Avoid the reflexive conclusion that all joint pain in older adults is the result of OA • Can develop in any joint that has suffered injury or other disease • Hallmark: cartilage degeneration Ø But not purely a degenerative disease; subchondral bone abnormalities and focal synovial inflammation are also seen in pathologic specimens 5 OA: DIAGNOSIS • Differential diagnosis: inflammatory and crystal arthritides, septic arthritis, bone pain due to malignancy • Bony enlargement and crepitus suggest OA Ø In the fingers, bony enlargement occurs in the distal interphalangeal joint (Heberden nodes) and in the proximal interphalangeal joints (Bouchard nodes) Ø Osteophytes are the radiographic counterpart of this enlargement, and asymmetric joint space narrowing is common • Joint tenderness and warmth may appear, but true synovitis -

Anatomy of the Ophthalmic Artery: Embryological Consideration
REVIEW ARTICLE doi: 10.2176/nmc.ra.2015-0324 Neurol Med Chir (Tokyo) 56, 585–591, 2016 Online June 8, 2016 Anatomy of the Ophthalmic Artery: Embryological Consideration Naoki TOMA1 1Department of Neurosurgery, Mie University Graduate School of Medicine, Tsu, Mie, Japan Abstract There are considerable variations in the anatomy of the human ophthalmic artery (OphA), such as anom- alous origins of the OphA and anastomoses between the OphA and the adjacent arteries. These anatomi- cal variations seem to attribute to complex embryology of the OphA. In human embryos and fetuses, primitive dorsal and ventral ophthalmic arteries (PDOphA and PVOphA) form the ocular branches, and the supraorbital division of the stapedial artery forms the orbital branches of the OphA, and then numerous anastomoses between the internal carotid artery (ICA) and the external carotid artery (ECA) systems emerge in connection with the OphA. These developmental processes can produce anatomical variations of the OphA, and we should notice these variations for neurosurgical and neurointerventional procedures. Key words: ophthalmic artery, anatomy, embryology, stapedial artery, primitive maxillary artery Introduction is to elucidate the anatomical variation of the OphA from the embryological viewpoint. The ophthalmic artery (OphA) consists of ocular and orbital branches. The ocular branches contribute to Embryology and Anatomy the blood supply of the optic apparatus, namely, the of the OphA optic nerve and the retina, and the orbital branches supply the optic adnexae, such -

Guideline-Management-Giant-Cell
Arthritis Care & Research Vol. 73, No. 8, August 2021, pp 1071–1087 DOI 10.1002/acr.24632 © 2021 American College of Rheumatology. This article has been contributed to by US Government employees and their work is in the public domain in the USA. 2021 American College of Rheumatology/Vasculitis Foundation Guideline for the Management of Giant Cell Arteritis and Takayasu Arteritis Mehrdad Maz,1 Sharon A. Chung,2 Andy Abril,3 Carol A. Langford,4 Mark Gorelik,5 Gordon Guyatt,6 Amy M. Archer,7 Doyt L. Conn,8 Kathy A. Full,9 Peter C. Grayson,10 Maria F. Ibarra,11 Lisa F. Imundo,5 Susan Kim,2 Peter A. Merkel,12 Rennie L. Rhee,12 Philip Seo,13 John H. Stone,14 Sangeeta Sule,15 Robert P. Sundel,16 Omar I. Vitobaldi,17 Ann Warner,18 Kevin Byram,19 Anisha B. Dua,7 Nedaa Husainat,20 Karen E. James,21 Mohamad A. Kalot,22 Yih Chang Lin,23 Jason M. Springer,1 Marat Turgunbaev,24 Alexandra Villa-Forte, 4 Amy S. Turner,24 and Reem A. Mustafa25 Guidelines and recommendations developed and/or endorsed by the American College of Rheumatology (ACR) are intended to provide guidance for particular patterns of practice and not to dictate the care of a particu- lar patient. The ACR considers adherence to the recommendations within this guideline to be voluntary, with the ultimate determination regarding their application to be made by the physician in light of each patient’s individual circumstances. Guidelines and recommendations are intended to promote beneficial or desirable outcomes but cannot guarantee any specific outcome. -

PMR) / Giant Cell Arteritis (GCA
Arthritis and Rheumatology Clinics of Kansas Patient Education Polymyalgic Rheumatica (PMR) / Giant Cell Arteritis (GCA) Introduction: PMR and GCA are related conditions affecting adults over the age of 50. Both are inflammatory diseases, with PMR involving the large joints of the hips and/or shoulders and GCA involving large and medium sized blood vessels. PMR occurs in about 30 individuals over the age of 50 per 100,000 population per year, while GCA is roughly half as common. Both conditions are about twice as common in women as in men and are seen most commonly in those of Northern European descent. The average age of onset is about 70 for both conditions, and with the aging population the prevalence of these disorders is expected to increase in the next few decades. While the cause of these conditions is unknown, the fact that they tend to occur in cooler climates and in clusters of cases every several years suggests that infections may trigger PMR and GCA. Having certain genes also seems to increase the risk of developing either of these disorders. Features of PMR: Patients with PMR tend to experience widespread pain in the regions of the shoulders, upper arms, neck, hips, buttock, and thighs. These symptoms may be sudden in onset and are accompanied by up to several hours of morning stiffness. While swelling of knees, elbows, or wrists may occur, there is most often no visible joint swelling but only difficulty with movement of the larger joints. A small percentage of patients may experience swelling of the entire hand and foot with edema, or excess fluid accumulation.