Anatomic and Embryologic Analysis of the Dural Branches of the Ophthalmic Artery
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Fundus Signs in Temporal Arteritis
Br J Ophthalmol: first published as 10.1136/bjo.62.9.591 on 1 September 1978. Downloaded from British Journal of Ophthalmology, 1978, 62, 591-594 Fundus signs in temporal arteritis D. McLEOD, E. 0. OJI, E. M. KOHNER, AND J. MARSHALL From Moorfields Eye Hospital and Institute of Ophthalmology, London SUMMARY A patient with temporal arteritis developed a variety of ischaemic lesions in the eyes. Infarction of the inner retina and optic nerve head was delineated on presentation by white swelling in the retinal nerve fibre layer. The role of interrupted axoplasmic transport in the production of this sign is discussed. Outer retinal infarction was also noted on presentation and subsequently gave rise to striking pigmented scars. Temporal arteritis often presents with visual loss, the central venous tributaries were of normal and necropsy examination in such cases shows wide- calibre and colour. No abnormality of the inner spread disease of the ophthalmic artery and the retina was noted in the territory of supply of the extraocular course of its ciliary and retinal branches central retinal artery. (Henkind et al., 1970). The medial and lateral At first sight the left eye showed a similar ophthal- posterior ciliary arteries supply the optic nerve head, moscopic picture, with pale swelling of the nasal the outer retina, and, in 20 to 50% of individuals, part of the optic disc and a row of fluffy white by copyright. a variable area of inner retina contiguous with the cotton-wool spots crossing the papillomacular optic disc (Hayreh, 1969); the central retinal artery bundle (Fig. 2). -

Case Report a Case of Incomplete Central Retinal Artery Occlusion Associated with Short Posterior Ciliary Artery Occlusion
Hindawi Publishing Corporation Case Reports in Ophthalmological Medicine Volume 2013, Article ID 105653, 4 pages http://dx.doi.org/10.1155/2013/105653 Case Report A Case of Incomplete Central Retinal Artery Occlusion Associated with Short Posterior Ciliary Artery Occlusion Shinji Makino, Mikiko Takezawa, and Yukihiro Sato Department of Ophthalmology, Jichi Medical University, 3311-1 Yakushiji, Tochigi, Shimotsuke 329-0498, Japan Correspondence should be addressed to Shinji Makino; [email protected] Received 12 December 2012; Accepted 1 January 2013 Academic Editors: S. Machida, M. B. Parodi, and P. Venkatesh Copyright © 2013 Shinji Makino et al. is is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. To our knowledge, incomplete central retinal artery occlusion associated with short posterior ciliary artery occlusion is extremely rare. Herein, we describe a case of a 62-year-old man who was referred to our hospital with of transient blindness in his right eye. At initial examination, the patient’s best-corrected visual acuity was 18/20 in the right eye. Fundus examination showed multiple so exudates around the optic disc and mild macular retinal edema in his right eye; however, a cherry red spot on the macula was not detected. Fluorescein angiography revealed delayed dye in�ow into the nasal choroidal hemisphere that is supplied by the short posterior ciliary artery. e following day, the patient’s visual acuity improved to 20/20. So exudates around the optic disc increased during observation and gradually disappeared. -

Microsurgical Anatomy of the Central Retinal Artery
Original Article Microsurgical Anatomy of the Central Retinal Artery Matias Baldoncini1,2, Alvaro Campero2,3, Gabriel Moran1, Maximiliano Avendan˜ o1, Pablo Hinojosa-Martı´nez1, Marcela Cimmino1, Pablo Buosi1, Valeria Forlizzi1, Joaquı´n Chuang1, Brian Gargurevich1 - BACKGROUND: The central retinal artery (CRA) has INTRODUCTION been described as one of the first branches of the he central retinal artery (CRA) is described as one of the ophthalmic artery.It arises medial to the ciliary ganglion first branches of the ophthalmic artery. It arises medial to and after a sinuous path within the orbital cavity it pene- T the ciliary ganglion, and after a sinuous path inside the trates the lower surface of the dura mater that covers the orbital cavity, penetrates the lower surface of the dura mater that optic nerve, approximately 1 cm behind the eyeball. How- covers the optic nerve, approximately 1 cm behind the eyeball. ever, the numerous anatomic descriptions that were made After a short journey inside this meninge, it crosses the cranial of the CRA have been insufficient or unclear in relation to nerve to be located in its center and travels until it reaches the optical papilla where it divides into several branches. During this certain characteristics that are analyzed in the present entire journey, the CRA does not present any anastomoses, study. considering it as a terminal branch.1-4 However, the descriptions that many investigators make about - METHODS: An electronic literature search was made in some of thesecharacteristics of the CRA differ with the classic the PubMed database and a cadaver dissection was per- disposition] (Tables 1e12).5-40 The numerous anatomic de- formed on 11 orbits fixed in formaldehyde. -

THE CENTRAL ARTERY of the RETINA*T H
Br J Ophthalmol: first published as 10.1136/bjo.44.5.280 on 1 May 1960. Downloaded from Brit. J. Ophthal. (1960) 44, 280. THE CENTRAL ARTERY OF THE RETINA*t H. A STUDY OF ITS DISTRIBUHTION AND ANASTOMOSES BY SOHAN SINGH AND RAMJI DASS Department ofAnatomy, Government Medical College, Patiala, India THERE is little unanimity regarding the distribution and anastomoses of the central artery of the retina. According to Frangois and Neetens (1954, 1956) and Fran9ois, Neetens, and Collette (1955), the central artery of the retina is completely devoid of branches, while according to Wolff (1939) and Behr (1935) it is free from branches only in its intraneural course. Magitot (1908), Quain (1909), Beauvieux and Ristitch (1924), Wolff (1940), Bignell (1952), Wybar (1956), and Steele and Blunt (1956) on the other hand demon- strated branches from all parts of its course. Most investigators agree that this artery contributes to the blood supply of the optic nerve, but Francois and others (1954, 1955, 1956) affirm that this is not the case. The presence of a central artery of the optic nerve described by Behr (1935), Wolff (1939, 1954), Francois and others (1954, 1955, 1956), and Wybar (1956), is denied by Beauvieux and Ristitch (1924) and Steele and Blunt (1956). Beauvieux and Ristitch (1924), Kershner (1943), Frangois and others http://bjo.bmj.com/ (1954, 1955, 1956), and Steele and Blunt (1956) say there are no anastomoses between the central retinal and other arteries, but Vail (1948), Wybar (1956), and several other authors have observed and described them. The lamina cribrosa is the only site at which these anastomoses have been studied in any detail, and in this case too, contradictory views have been expressed. -

Neurovascular Anatomy (1): Anterior Circulation Anatomy
Neurovascular Anatomy (1): Anterior Circulation Anatomy Natthapon Rattanathamsakul, MD. December 14th, 2017 Contents: Neurovascular Anatomy Arterial supply of the brain . Anterior circulation . Posterior circulation Arterial supply of the spinal cord Venous system of the brain Neurovascular Anatomy (1): Anatomy of the Anterior Circulation Carotid artery system Ophthalmic artery Arterial circle of Willis Arterial territories of the cerebrum Cerebral Vasculature • Anterior circulation: Internal carotid artery • Posterior circulation: Vertebrobasilar system • All originates at the arch of aorta Flemming KD, Jones LK. Mayo Clinic neurology board review: Basic science and psychiatry for initial certification. 2015 Common Carotid Artery • Carotid bifurcation at the level of C3-4 vertebra or superior border of thyroid cartilage External carotid artery Supply the head & neck, except for the brain the eyes Internal carotid artery • Supply the brain the eyes • Enter the skull via the carotid canal Netter FH. Atlas of human anatomy, 6th ed. 2014 Angiographic Correlation Uflacker R. Atlas of vascular anatomy: an angiographic approach, 2007 External Carotid Artery External carotid artery • Superior thyroid artery • Lingual artery • Facial artery • Ascending pharyngeal artery • Posterior auricular artery • Occipital artery • Maxillary artery • Superficial temporal artery • Middle meningeal artery – epidural hemorrhage Netter FH. Atlas of human anatomy, 6th ed. 2014 Middle meningeal artery Epidural hematoma http://www.jrlawfirm.com/library/subdural-epidural-hematoma -

Download PDF File
ONLINE FIRST This is a provisional PDF only. Copyedited and fully formatted version will be made available soon. ISSN: 0015-5659 e-ISSN: 1644-3284 Two cases of combined anatomical variations: maxillofacial trunk, vertebral, posterior communicating and anterior cerebral atresia, linguofacial and labiomental trunks Authors: M. C. Rusu, A. M. Jianu, M. D. Monea, A. C. Ilie DOI: 10.5603/FM.a2021.0007 Article type: Case report Submitted: 2020-11-28 Accepted: 2021-01-08 Published online: 2021-01-29 This article has been peer reviewed and published immediately upon acceptance. It is an open access article, which means that it can be downloaded, printed, and distributed freely, provided the work is properly cited. Articles in "Folia Morphologica" are listed in PubMed. Powered by TCPDF (www.tcpdf.org) Two cases of combined anatomical variations: maxillofacial trunk, vertebral, posterior communicating and anterior cerebral atresia, linguofacial and labiomental trunks M.C. Rusu et al., The maxillofacial trunk M.C. Rusu1, A.M. Jianu2, M.D. Monea2, A.C. Ilie3 1Division of Anatomy, Faculty of Dental Medicine, “Carol Davila” University of Medicine and Pharmacy, Bucharest, Romania 2Department of Anatomy, Faculty of Medicine, “Victor Babeş” University of Medicine and Pharmacy, Timişoara, Romania 3Department of Functional Sciences, Discipline of Public Health, Faculty of Medicine, “Victor Babes” University of Medicine and Pharmacy, Timisoara, Romania Address for correspondence: M.C. Rusu, MD, PhD (Med.), PhD (Biol.), Dr. Hab., Prof., Division of Anatomy, Faculty of Dental Medicine, “Carol Davila” University of Medicine and Pharmacy, 8 Eroilor Sanitari Blvd., RO-76241, Bucharest, Romania, , tel: +40722363705 e-mail: [email protected] ABSTRACT Background: Commonly, arterial anatomic variants are reported as single entities. -

Endovascular Approach to Ruptured Sphenopalatine Artery: a Case Report and Literature Review
J Spine Res Surg 2021; 3 (2): 037-044 DOI: 10.26502/fjsrs0028 Case Report Endovascular Approach to Ruptured Sphenopalatine Artery: A Case Report and Literature Review Ram Saha1, Abu Bakar Siddik2, Masum Rahman3, Samar Ikram3, Cecile Riviere-cazaux4, Abdullah Alamgir5, Badrul Alam Mondal6, Quazi Deen Mohammad6, Sirajee Shafiqul Islam 7* 1Department of Neurology, Virginia Commonwealth University, VA, USA 2Department of Pain Medicine, Mayo Clinic, Jacksonville, Florida, USA 3Department of Neurological Surgery, Mayo Clinic, MN, USA 4Mayo Clinic Alix school of medicine, Rochester, MN, USA 5Department of Neurosurgery, National Institute of Neurosciences & Hospital, Dhaka, Bangladesh 6Department of Neurology, National Institute of Neurosciences & Hospital, Dhaka, Bangladesh 7Department of Interventional Neurology, National Institute of Neurosciences & Hospital, Dhaka, Bangladesh *Corresponding Author: Dr. Sirajee Shafiqul Islam, Associate Professor, Department of Interventional Neurology, National Institute of Neurosciences & Hospital, Dhaka, Bangladesh Received: 14 May 2021; Accepted: 25 May 2021; Published: 31 May 2021 Citation: Ram Saha, Abu Bakar Siddik, Masum Rahman, Samar Ikram, Cecile Riviere-cazaux, Abdullah Alamgir, Badrul Alam Mondal, Quazi Deen Mohammad, Sirajee Shafiqul Islam. Endovascular Approach to Ruptured Sphenopalatine Artery: A Case Report and Literature Review. Journal of Spine Research and Surgery 3 (2021): 037- 044. Abstract Epistaxis is a rare complication following the endonasal skull-base chordoma through an endonasal approach. approach of skull base surgery. Conservative methods An endovascular catheter digital subtraction angiogram like anterior and posterior nasal packing can be useful, identified the cause of epistaxis as a rupture of the left but when these fail, a neuro-interventional technique sphenopalatine artery branch of the left external carotid can be used as a last-resort measure in cases of severe artery. -
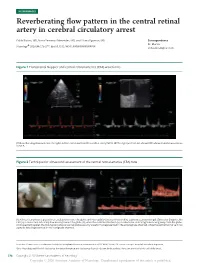
Reverberating Flow Pattern in the Central Retinal Artery in Cerebral
NEUROIMAGES Reverberating flow pattern in the central retinal artery in cerebral circulatory arrest Pablo Blanco, MD, Mar´ıa Fernanda Men´endez, MD, and Liliana Figueroa, MD Correspondence Dr. Blanco Neurology 2020;94:276-277. doi:10.1212/WNL.0000000000008918 ® [email protected] Figure 1 Transcranial Doppler and central retinal arteries (CRA) waveforms (A) Reverberating flow pattern in the right (and left, not shown) middle cerebral artery (MCA). (B) The right (and left, not shown) CRA showed similar waveforms to MCA. Figure 2 Technique for ultrasound assessment of the central retinal arteries (CRA) flow (A) A linear transducer is placed in an axial position over the globe, with the eyelids closed and covered by a generous amount of gel. (B) In color Doppler, the CRA (a) is coded red, indicating flow moving toward the globe (G), while the central retinal vein (v) is coded blue, indicating flow moving away from the globe. (C) In spectral Doppler, the CRA typically shows low resistance velocity waveforms (represented in the anterograde channel), while the central retinal vein has a phasic flow (represented in the retrograde channel). From the “Centro Unico´ Coordinador de Ablacion´ e Implante Provincia de Buenos Aires (CUCAIBA)” Team, “Dr. Emilio Ferreyra” Hospital, Necochea, Argentina. Go to Neurology.org/N for full disclosures. Funding information and disclosures deemed relevant by the authors, if any, are provided at the end of the article. 276 Copyright © 2020 American Academy of Neurology Copyright © 2020 American Academy of Neurology. Unauthorized reproduction of this article is prohibited. A 49-year-old woman developed signs of brain death after when obtaining flow signals through the cranial bone is not a severe traumatic brain injury. -

Clinical Importance of the Middle Meningeal Artery
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Jagiellonian Univeristy Repository FOLIA MEDICA CRACOVIENSIA 41 Vol. LIII, 1, 2013: 41–46 PL ISSN 0015-5616 Przemysław Chmielewski1, Janusz skrzat1, Jerzy waloCha1 CLINICAL IMPORTANCE OF THE MIDDLE MENINGEAL ARTERY Abstract: Middle meningeal artery (MMA)is an important branch which supplies among others cranial dura mater. It directly attaches to the cranial bones (is incorporated into periosteal layer of dura mater), favors common injuries in course of head trauma. This review describes available data on the MMA considering its varability, or treats specific diseases or injuries where the course of MMA may have clinical impact. Key words: Middle meningeal artery (MMA), aneurysm of the middle meningeal artery, epidural he- matoma, anatomical variation of MMA. TOPOGRAPHY OF THE MIDDLE MENINGEAL ARTERY AND ITS BRANCHES Middle meningeal artery (MMA) [1] is most commonly the strongest branch of maxillary artery (from external carotid artery) [2]. It supplies blood to cranial dura mater, and through the numerous perforating branches it nourishes also periosteum of the inner aspect of cranial bones. It enters the middle cranial fossa through the foramen spinosum, and courses between the dura mater and the inner aspect of the vault of the skull. Next it divides into two terminal branches — frontal (anterior) which supplies blood to bones forming anterior cranial fossa and the anterior part of the middle cranial fossa; parietal branch (posterior), which runs more horizontally toward the back and supplies posterior part of the middle cranial fossa and supratentorial part of the posterior cranial fossa. -

LETTERS to the EDITOR. Middle Cerebral Artery Tortuosity Associated
J Neurosurg 130:1763–1788, 2019 Neurosurgical Forum LETTERS TO THE EDITOR Middle cerebral artery tortuosity tective factors against aneurysm formation.” Nevertheless, according to that explanation, it can be inferred that the associated with aneurysm incidence of aneurysms in patients with local tortuosity development should be decreased rather than increased. Therefore, it would be better for the authors to provide an in-depth ex- planation about the above results and arguments. TO THE EDITOR: We read with great interest the ar- Second, the authors stated, “There are a few rare ge- ticle by Kliś et al.2 (Kliś KM, Krzyżewski RM, Kwinta netic syndromes that are linked to the presence of vessel BM, et al: Computer-aided analysis of middle cerebral tortuosity, such as artery tortuosity syndrome or Loeys- artery tortuosity: association with aneurysm develop- Dietz syndrome.” The genetic syndromes they mention ment. J Neurosurg [epub ahead of print May 18, 2018; are systemic lesions involving multiple parts of vessels DOI: 10.3171/2017.12.JNS172114]). The authors conclude of the body and therefore often involve multiple intracra- that “an increased deviation of the middle cerebral artery nial aneurysms.1,3,5 However, this article did not provide (MCA) from a straight axis (described by relative length detailed information on the characteristics of intracranial [RL]), a decreased sum of all MCA angles (described by aneurysms, for example, the incidence of multiple aneu- sum of angle metrics [SOAM]), a local increase of the rysms, the specific sites of the MCA aneurysms (M1, M2, MCA angle heterogeneity, and an increase in changes in M3, M4), and the size of the aneurysms, etc. -

The Ophthalmic Artery Ii
Brit. J. Ophthal. (1962) 46, 165. THE OPHTHALMIC ARTERY II. INTRA-ORBITAL COURSE* BY SOHAN SINGH HAYREHt AND RAMJI DASS Government Medical College, Patiala, India Material THIS study was carried out in 61 human orbits obtained from 38 dissection- room cadavers. In 23 cadavers both the orbits were examined, and in the remaining fifteen only one side was studied. With the exception of three cadavers of children aged 4, 11, and 12 years, the specimens were from old persons. Method Neoprene latex was injected in situ, either through the internal carotid artery or through the most proximal part of the ophthalmic artery, after opening the skull and removing the brain. The artery was first irrigated with water. After injection the part was covered with cotton wool soaked in 10 per cent. formalin for from 24 to 48 hours to coagulate the latex. The roof of the orbit was then opened and the ophthalmic artery was carefully studied within the orbit. Observations COURSE For descriptive purposes the intra-orbital course of the ophthalmic artery has been divided into three parts (Singh and Dass, 1960). (1) The first part extends from the point of entrance of the ophthalmic artery into the orbit to the point where the artery bends to become the second part. This part usually runs along the infero-lateral aspect of the optic nerve. (2) The second part crosses over or under the optic nerve running in a medial direction from the infero-lateral to the supero-medial aspect of the nerve. (3) The thirdpart extends from the point at which the second part bends at the supero-medial aspect of the optic nerve to its termination. -

Head & Neck Muscle Table
Robert Frysztak, PhD. Structure of the Human Body Loyola University Chicago Stritch School of Medicine HEAD‐NECK MUSCLE TABLE PROXIMAL ATTACHMENT DISTAL ATTACHMENT MUSCLE INNERVATION MAIN ACTIONS BLOOD SUPPLY MUSCLE GROUP (ORIGIN) (INSERTION) Anterior floor of orbit lateral to Oculomotor nerve (CN III), inferior Abducts, elevates, and laterally Inferior oblique Lateral sclera deep to lateral rectus Ophthalmic artery Extra‐ocular nasolacrimal canal division rotates eyeball Inferior aspect of eyeball, posterior to Oculomotor nerve (CN III), inferior Depresses, adducts, and laterally Inferior rectus Common tendinous ring Ophthalmic artery Extra‐ocular corneoscleral junction division rotates eyeball Lateral aspect of eyeball, posterior to Lateral rectus Common tendinous ring Abducent nerve (CN VI) Abducts eyeball Ophthalmic artery Extra‐ocular corneoscleral junction Medial aspect of eyeball, posterior to Oculomotor nerve (CN III), inferior Medial rectus Common tendinous ring Adducts eyeball Ophthalmic artery Extra‐ocular corneoscleral junction division Passes through trochlea, attaches to Body of sphenoid (above optic foramen), Abducts, depresses, and medially Superior oblique superior sclera between superior and Trochlear nerve (CN IV) Ophthalmic artery Extra‐ocular medial to origin of superior rectus rotates eyeball lateral recti Superior aspect of eyeball, posterior to Oculomotor nerve (CN III), superior Elevates, adducts, and medially Superior rectus Common tendinous ring Ophthalmic artery Extra‐ocular the corneoscleral junction division