Predicted Coronavirus Nsp5 Protease Cleavage Sites in The
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
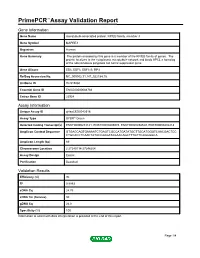
Primepcr™Assay Validation Report
PrimePCR™Assay Validation Report Gene Information Gene Name microtubule-associated protein, RP/EB family, member 3 Gene Symbol MAPRE3 Organism Human Gene Summary The protein encoded by this gene is a member of the RP/EB family of genes. The protein localizes to the cytoplasmic microtubule network and binds APCL a homolog of the adenomatous polyposis coli tumor suppressor gene. Gene Aliases EB3, EBF3, EBF3-S, RP3 RefSeq Accession No. NC_000002.11, NT_022184.15 UniGene ID Hs.515860 Ensembl Gene ID ENSG00000084764 Entrez Gene ID 22924 Assay Information Unique Assay ID qHsaCED0042518 Assay Type SYBR® Green Detected Coding Transcript(s) ENST00000233121, ENST00000405074, ENST00000458529, ENST00000402218 Amplicon Context Sequence GTGACCAGTGAAAATCTGAGTCGCCATGATATGCTTGCATGGGTCAACGACTCC CTGCACCTCAACTATACCAAGATAGAACAGCTTTGTTCAGGGGCA Amplicon Length (bp) 69 Chromosome Location 2:27245114-27246204 Assay Design Exonic Purification Desalted Validation Results Efficiency (%) 90 R2 0.9993 cDNA Cq 24.76 cDNA Tm (Celsius) 80 gDNA Cq 26.8 Specificity (%) 100 Information to assist with data interpretation is provided at the end of this report. Page 1/4 PrimePCR™Assay Validation Report MAPRE3, Human Amplification Plot Amplification of cDNA generated from 25 ng of universal reference RNA Melt Peak Melt curve analysis of above amplification Standard Curve Standard curve generated using 20 million copies of template diluted 10-fold to 20 copies Page 2/4 PrimePCR™Assay Validation Report Products used to generate validation data Real-Time PCR Instrument CFX384 Real-Time PCR Detection System Reverse Transcription Reagent iScript™ Advanced cDNA Synthesis Kit for RT-qPCR Real-Time PCR Supermix SsoAdvanced™ SYBR® Green Supermix Experimental Sample qPCR Human Reference Total RNA Data Interpretation Unique Assay ID This is a unique identifier that can be used to identify the assay in the literature and online. -

Targeting Lineage-Specific MITF Pathway in Human Melanoma Cell Lines by A-485, the Selective Small Molecule Inhibitor of P300/CBP
Author Manuscript Published OnlineFirst on September 28, 2018; DOI: 10.1158/1535-7163.MCT-18-0511 Author manuscripts have been peer reviewed and accepted for publication but have not yet been edited. Targeting lineage-specific MITF pathway in human melanoma cell lines by A-485, the selective small molecule inhibitor of p300/CBP Rui Wang1,2, Yupeng He1, Valerie Robinson1, Ziping Yang1, Paul Hessler1, Loren M. Lasko1, Xin Lu1, Anahita Bhathena1, Albert Lai1, Tamar Uziel1, Lloyd T. Lam1,3 1Oncology Discovery, AbbVie, 1 N Waukegan Road, North Chicago, IL 60064-6101, USA. 2Former AbbVie employee Running title: p300/CBP inhibition targets MITF in melanoma cell lines Keywords: p300/CBP inhibitor, melanoma, MITF, A-485, senescence Word count: 3,040 (excluding the abstract, references and legends) References: 29 3Correspondence: Lloyd T. Lam, Oncology Discovery, AP10-214, Dept. R4CD 1 North Waukegan Road North Chicago, IL 60064-6098, USA. Phone: 847-937-5585 Fax: 847-935-4994 E-mail: [email protected] Financial information Y.H., V.R., Z.Y., P.H., L.M.L., X.L, A.B., A.L., T.U., L.T.L. are employees of AbbVie. R.W. was an employee of AbbVie at the time of the study. The design, study conduct, and financial support for this research were provided by AbbVie. AbbVie participated in the interpretation of data, review, and approval of the manuscript. R.W., Y.H., V.R., Z.Y., P.H., L.M.L., X.L, A.B., A.L., T.U., L.T.L. have nothing to disclose. Downloaded from mct.aacrjournals.org on September 27, 2021. -

Supplementary Table 3 Complete List of RNA-Sequencing Analysis of Gene Expression Changed by ≥ Tenfold Between Xenograft and Cells Cultured in 10%O2
Supplementary Table 3 Complete list of RNA-Sequencing analysis of gene expression changed by ≥ tenfold between xenograft and cells cultured in 10%O2 Expr Log2 Ratio Symbol Entrez Gene Name (culture/xenograft) -7.182 PGM5 phosphoglucomutase 5 -6.883 GPBAR1 G protein-coupled bile acid receptor 1 -6.683 CPVL carboxypeptidase, vitellogenic like -6.398 MTMR9LP myotubularin related protein 9-like, pseudogene -6.131 SCN7A sodium voltage-gated channel alpha subunit 7 -6.115 POPDC2 popeye domain containing 2 -6.014 LGI1 leucine rich glioma inactivated 1 -5.86 SCN1A sodium voltage-gated channel alpha subunit 1 -5.713 C6 complement C6 -5.365 ANGPTL1 angiopoietin like 1 -5.327 TNN tenascin N -5.228 DHRS2 dehydrogenase/reductase 2 leucine rich repeat and fibronectin type III domain -5.115 LRFN2 containing 2 -5.076 FOXO6 forkhead box O6 -5.035 ETNPPL ethanolamine-phosphate phospho-lyase -4.993 MYO15A myosin XVA -4.972 IGF1 insulin like growth factor 1 -4.956 DLG2 discs large MAGUK scaffold protein 2 -4.86 SCML4 sex comb on midleg like 4 (Drosophila) Src homology 2 domain containing transforming -4.816 SHD protein D -4.764 PLP1 proteolipid protein 1 -4.764 TSPAN32 tetraspanin 32 -4.713 N4BP3 NEDD4 binding protein 3 -4.705 MYOC myocilin -4.646 CLEC3B C-type lectin domain family 3 member B -4.646 C7 complement C7 -4.62 TGM2 transglutaminase 2 -4.562 COL9A1 collagen type IX alpha 1 chain -4.55 SOSTDC1 sclerostin domain containing 1 -4.55 OGN osteoglycin -4.505 DAPL1 death associated protein like 1 -4.491 C10orf105 chromosome 10 open reading frame 105 -4.491 -

Identification of Differentially Expressed
IDENTIFICATION OF DIFFERENTIALLY EXPRESSED TRANSCRIPTS OF Tdrd7 NULL MUTANT MOUSE LENS by Shaili D. Patel A thesis submitted to the Faculty of the University of Delaware in partial fulfillment of the requirements for the degree of Degree in Biological Sciences in Arts and Sciences with Distinction Spring 2014 Copyrights 2014 Shaili D. Patel All Rights Reserved IDENTIFICATION OF DIFFERENTIALLY EXPRESSED TRANSCRIPTS OF Tdrd7 NULL MUTANT MOUSE LENS by Shaili D. Patel Approved: __________________________________________________________ Dr. Salil A. Lachke, PhD., Professor in charge of thesis on behalf of the Advisory Committee Approved: __________________________________________________________ Dr. Jeffery L. Caplan, PhD., Committee member from the Department of Delaware Biotechnology Institute Approved: __________________________________________________________ Dr. Rolf Joerger, PhD., Committee member from the Board of Senior Thesis Readers Approved: __________________________________________________________ Michelle Provost-Craig, Ph.D. Chair of the University Committee on Student and Faculty Honors ACKNOWLEDGMENTS First and foremost, I would like to thank Dr. Lachke, without whom this project would’ve been impossible. Thank you soooo much Dr. Lachke, I really hope I can be ten percent of what you are as a person. Additionally, I want to thank you for making such a big difference in my career, I am very thankful for that. Thank you mom and daddy for the roots and wings. Thank you Parth for being there every single day when I was finishing up my experiments and thesis, you were a great mental support and for being such a good friend. I would specially like to thank Carrie Barnum, who trained me in this lab. I will be taking all these lab skills to where I go in future, thanks a ton for that Carrie, and thank you for the little treats every once in a while; they were a great power booster J. -

The Tudor Domain Protein Tapas, a Homolog of the Vertebrate Tdrd7, Functions in the Pirna Pathway to Regulate Retrotransposons in Germline of Drosophila Melanogaster
This document is downloaded from DR‑NTU (https://dr.ntu.edu.sg) Nanyang Technological University, Singapore. The Tudor domain protein Tapas, a homolog of the vertebrate Tdrd7, functions in the piRNA pathway to regulate retrotransposons in germline of Drosophila melanogaster Patil, Veena S.; Anand, Amit; Chakrabarti, Alisha; Kai, Toshie 2014 Patil, V. S., Anand, A., Chakrabarti, A., & Kai, T. (2014). The Tudor domain protein Tapas, a homolog of the vertebrate Tdrd7, functions in the piRNA pathway to regulate retrotransposons in germline of Drosophila melanogaster. BMC Biology, 12, 61‑. https://hdl.handle.net/10356/84728 https://doi.org/10.1186/s12915‑014‑0061‑9 © 2014 Patil et al.; licensee BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly credited. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated. Downloaded on 25 Sep 2021 01:20:27 SGT Patil et al. BMC Biology 2014, 12:61 http://www.biomedcentral.com/1741-7007/12/61 RESEARCH ARTICLE Open Access The Tudor domain protein Tapas, a homolog of the vertebrate Tdrd7, functions in the piRNA pathway to regulate retrotransposons in germline of Drosophila melanogaster Veena S Patil1,4†, Amit Anand1†, Alisha Chakrabarti1,3 and Toshie Kai1,2* Abstract Background: Piwi-interacting RNAs (piRNAs) are a special class of small RNAs that provide defense against transposable elements in animal germline cells. -

Arabidopsis SMG7 Protein Is Required for Exit from Meiosis
2208 Research Article Arabidopsis SMG7 protein is required for exit from meiosis Nina Riehs1,*, Svetlana Akimcheva1,*, Jasna Puizina1,‡, Petra Bulankova1, Rachel A. Idol2,§, Jiri Siroky3, Alexander Schleiffer4, Dieter Schweizer1, Dorothy E. Shippen2 and Karel Riha1,¶ 1Gregor Mendel Institute of Molecular Plant Biology, Austrian Academy of Sciences, Dr Bohr-Gasse 3, 1030 Vienna, Austria 2Department of Biochemistry and Biophysics, Texas A&M University, College Station, TX 77843-2128, USA 3Institute of Biophysics, Czech Academy of Sciences, 612 65 Brno, Czech Republic 4Research Institute of Molecular Pathology, 1030 Vienna, Austria *These authors contributed equally to this work ‡Present address: Department of Biology, University of Split, Teslina 12, Croatia §Present address: Department of Internal Medicine, Division of Hematology, Division of Laboratory Medicine, Washington University School of Medicine, St Louis, MO 63110, USA ¶Author for correspondence (e-mail: [email protected]) Accepted 9 April 2008 Journal of Cell Science 121, 2208-2216 Published by The Company of Biologists 2008 doi:10.1242/jcs.027862 Summary Meiosis consists of two nuclear divisions that are separated by that is characterized by delayed chromosome decondensation a short interkinesis. Here we show that the SMG7 protein, which and aberrant rearrangement of the meiotic spindle. The smg7 plays an evolutionarily conserved role in nonsense-mediated phenotype was mimicked by exposing meiocytes to the RNA decay (NMD) in animals and yeast, is essential for the proteasome inhibitor MG115. Together, these data indicate that progression from anaphase to telophase in the second meiotic SMG7 counteracts cyclin-dependent kinase (CDK) activity at division in Arabidopsis. Arabidopsis SMG7 is an essential gene, the end of meiosis, and reveal a novel link between SMG7 and the disruption of which causes embryonic lethality. -

Gene Section Review
Atlas of Genetics and Cytogenetics in Oncology and Haematology OPEN ACCESS JOURNAL AT INIST-CNRS Gene Section Review TACC1 (transforming, acidic coiled-coil containing protein 1) Ivan Still, Melissa R Eslinger, Brenda Lauffart Department of Biological Sciences, Arkansas Tech University, 1701 N Boulder Ave Russellville, AR 72801, USA (IS), Department of Chemistry and Life Science Bartlett Hall, United States Military Academy, West Point, New York 10996, USA (MRE), Department of Physical Sciences Arkansas Tech University, 1701 N Boulder Ave Russellville, AR 72801, USA (BL) Published in Atlas Database: December 2008 Online updated version : http://AtlasGeneticsOncology.org/Genes/TACC1ID42456ch8p11.html DOI: 10.4267/2042/44620 This work is licensed under a Creative Commons Attribution-Noncommercial-No Derivative Works 2.0 France Licence. © 2009 Atlas of Genetics and Cytogenetics in Oncology and Haematology Identity Note: - AK304507 and AK303596 sequences may be suspect Other names: Ga55; DKFZp686K18126; KIAA1103 (see UCSC Genome Bioinformatics Site HGNC (Hugo): TACC1 (http://genome.ucsc.edu) for more details. - Transcript/isoform nomenclature as per Line et al, Location: 8p11.23 2002 and Lauffart et al., 2006. TACC1F transcript Note: This gene has three proposed transcription start includes exon 1, 2 and 3 (correction to Fig 6 of Lauffart sites beginning at 38763938 bp, 38733914 bp, et al., 2006). 38705165 bp from pter. Pseudogene DNA/RNA Partially processed pseudogene: - 91% identity corresponding to base 596 to 2157 of Description AF049910. The gene is composed of 19 exons spanning 124.5 kb. Location: 10p11.21. Location base pair: starts at 37851943 and ends at Transcription 37873633 from pter (according to hg18-March_2006). -

EB3 (MAPRE3) (NM 012326) Human Tagged ORF Clone Product Data
OriGene Technologies, Inc. 9620 Medical Center Drive, Ste 200 Rockville, MD 20850, US Phone: +1-888-267-4436 [email protected] EU: [email protected] CN: [email protected] Product datasheet for RC204135 EB3 (MAPRE3) (NM_012326) Human Tagged ORF Clone Product data: Product Type: Expression Plasmids Product Name: EB3 (MAPRE3) (NM_012326) Human Tagged ORF Clone Tag: Myc-DDK Symbol: MAPRE3 Synonyms: EB3; EBF3; EBF3-S; RP3 Vector: pCMV6-Entry (PS100001) E. coli Selection: Kanamycin (25 ug/mL) Cell Selection: Neomycin ORF Nucleotide >RC204135 ORF sequence Sequence: Red=Cloning site Blue=ORF Green=Tags(s) TTTTGTAATACGACTCACTATAGGGCGGCCGGGAATTCGTCGACTGGATCCGGTACCGAGGAGATCTGCC GCCGCGATCGCC ATGGCCGTCAATGTGTACTCCACATCTGTGACCAGTGAAAATCTGAGTCGCCATGATATGCTTGCATGGG TCAACGACTCCCTGCACCTCAACTATACCAAGATAGAACAGCTTTGTTCAGGGGCAGCCTACTGCCAGTT CATGGACATGCTCTTCCCCGGCTGTGTGCACTTGAGGAAAGTGAAGTTCCAGGCCAAACTAGAGCATGAA TACATCCACAACTTCAAGGTGCTGCAAGCAGCTTTCAAGAAGATGGGTGTTGACAAAATCATTCCTGTAG AGAAATTAGTGAAAGGAAAATTCCAAGATAATTTTGAGTTTATTCAGTGGTTTAAGAAATTCTTTGACGC AAACTATGATGGAAAGGATTACAACCCTCTGCTGGCGCGGCAGGGCCAGGACGTAGCGCCACCTCCTAAC CCAGGTGATCAGATCTTCAACAAATCCAAGAAACTCATTGGCACAGCAGTTCCACAGAGGACGTCCCCCA CAGGCCCAAAAAACATGCAGACCTCTGGCCGGCTGAGCAATGTGGCCCCCCCCTGCATTCTCCGGAAGAA TCCTCCATCAGCCCGAAATGGCGGCCATGAGACTGATGCCCAAATTCTTGAACTCAACCAACAGCTGGTG GACTTGAAGCTGACAGTGGATGGGCTGGAGAAGGAACGTGACTTCTACTTCAGCAAACTTCGTGACATCG AGCTCATCTGCCAGGAGCATGAAAGTGAAAACAGCCCTGTTATCTCAGGCATCATTGGCATCCTCTATGC CACAGAGGAAGGATTCGCACCCCCTGAGGACGATGAGATTGAAGAGCATCAACAAGAAGACCAGGACGAG TAC ACGCGTACGCGGCCGCTCGAGCAGAAACTCATCTCAGAAGAGGATCTGGCAGCAAATGATATCCTGGATT -

Supporting Information
Supporting Information Pouryahya et al. SI Text Table S1 presents genes with the highest absolute value of Ricci curvature. We expect these genes to have significant contribution to the network’s robustness. Notably, the top two genes are TP53 (tumor protein 53) and YWHAG gene. TP53, also known as p53, it is a well known tumor suppressor gene known as the "guardian of the genome“ given the essential role it plays in genetic stability and prevention of cancer formation (1, 2). Mutations in this gene play a role in all stages of malignant transformation including tumor initiation, promotion, aggressiveness, and metastasis (3). Mutations of this gene are present in more than 50% of human cancers, making it the most common genetic event in human cancer (4, 5). Namely, p53 mutations play roles in leukemia, breast cancer, CNS cancers, and lung cancers, among many others (6–9). The YWHAG gene encodes the 14-3-3 protein gamma, a member of the 14-3-3 family proteins which are involved in many biological processes including signal transduction regulation, cell cycle pro- gression, apoptosis, cell adhesion and migration (10, 11). Notably, increased expression of 14-3-3 family proteins, including protein gamma, have been observed in a number of human cancers including lung and colorectal cancers, among others, suggesting a potential role as tumor oncogenes (12, 13). Furthermore, there is evidence that loss Fig. S1. The histogram of scalar Ricci curvature of 8240 genes. Most of the genes have negative scalar Ricci curvature (75%). TP53 and YWHAG have notably low of p53 function may result in upregulation of 14-3-3γ in lung cancer Ricci curvatures. -

Aneuploidy: Using Genetic Instability to Preserve a Haploid Genome?
Health Science Campus FINAL APPROVAL OF DISSERTATION Doctor of Philosophy in Biomedical Science (Cancer Biology) Aneuploidy: Using genetic instability to preserve a haploid genome? Submitted by: Ramona Ramdath In partial fulfillment of the requirements for the degree of Doctor of Philosophy in Biomedical Science Examination Committee Signature/Date Major Advisor: David Allison, M.D., Ph.D. Academic James Trempe, Ph.D. Advisory Committee: David Giovanucci, Ph.D. Randall Ruch, Ph.D. Ronald Mellgren, Ph.D. Senior Associate Dean College of Graduate Studies Michael S. Bisesi, Ph.D. Date of Defense: April 10, 2009 Aneuploidy: Using genetic instability to preserve a haploid genome? Ramona Ramdath University of Toledo, Health Science Campus 2009 Dedication I dedicate this dissertation to my grandfather who died of lung cancer two years ago, but who always instilled in us the value and importance of education. And to my mom and sister, both of whom have been pillars of support and stimulating conversations. To my sister, Rehanna, especially- I hope this inspires you to achieve all that you want to in life, academically and otherwise. ii Acknowledgements As we go through these academic journeys, there are so many along the way that make an impact not only on our work, but on our lives as well, and I would like to say a heartfelt thank you to all of those people: My Committee members- Dr. James Trempe, Dr. David Giovanucchi, Dr. Ronald Mellgren and Dr. Randall Ruch for their guidance, suggestions, support and confidence in me. My major advisor- Dr. David Allison, for his constructive criticism and positive reinforcement. -

Intrinsic Disorder in Tetratricopeptide Repeat Proteins
International Journal of Molecular Sciences Article Intrinsic Disorder in Tetratricopeptide Repeat Proteins 1, 1, 1, 2 Nathan W. Van Bibber y, Cornelia Haerle y, Roy Khalife y, Bin Xue and Vladimir N. Uversky 1,3,4,* 1 Department of Molecular Medicine Morsani College of Medicine, University of South Florida, 12901 Bruce B. Downs Blvd., Tampa, FL 33612, USA; [email protected] (N.W.V.B.); [email protected] (C.H.); [email protected] (R.K.) 2 Department of Cell Biology, Microbiology and Molecular Biology, School of Natural Sciences and Mathematics, College of Arts and Sciences, University of South Florida, Tampa, FL 33620, USA; [email protected] 3 USF Health Byrd Alzheimer’s Research Institute, Morsani College of Medicine, University of South Florida, 12901 Bruce B. Downs Blvd., Tampa, FL 33612, USA 4 Institute for Biological Instrumentation, Russian Academy of Sciences, Federal Research Center “Pushchino Scientific Center for Biological Research of the Russian Academy of Sciences”, 4 Institutskaya St., Pushchino, 142290 Moscow Region, Russia * Correspondence: [email protected]; Tel.: +1-813-974-5816; Fax: +1-813-974-7357 These authors contributed equally to this work. y Received: 21 April 2020; Accepted: 22 May 2020; Published: 25 May 2020 Abstract: Among the realm of repeat containing proteins that commonly serve as “scaffolds” promoting protein-protein interactions, there is a family of proteins containing between 2 and 20 tetratricopeptide repeats (TPRs), which are functional motifs consisting of 34 amino acids. The most distinguishing feature of TPR domains is their ability to stack continuously one upon the other, with these stacked repeats being able to affect interaction with binding partners either sequentially or in combination. -

In This Table Protein Name, Uniprot Code, Gene Name P-Value
Supplementary Table S1: In this table protein name, uniprot code, gene name p-value and Fold change (FC) for each comparison are shown, for 299 of the 301 significantly regulated proteins found in both comparisons (p-value<0.01, fold change (FC) >+/-0.37) ALS versus control and FTLD-U versus control. Two uncharacterized proteins have been excluded from this list Protein name Uniprot Gene name p value FC FTLD-U p value FC ALS FTLD-U ALS Cytochrome b-c1 complex P14927 UQCRB 1.534E-03 -1.591E+00 6.005E-04 -1.639E+00 subunit 7 NADH dehydrogenase O95182 NDUFA7 4.127E-04 -9.471E-01 3.467E-05 -1.643E+00 [ubiquinone] 1 alpha subcomplex subunit 7 NADH dehydrogenase O43678 NDUFA2 3.230E-04 -9.145E-01 2.113E-04 -1.450E+00 [ubiquinone] 1 alpha subcomplex subunit 2 NADH dehydrogenase O43920 NDUFS5 1.769E-04 -8.829E-01 3.235E-05 -1.007E+00 [ubiquinone] iron-sulfur protein 5 ARF GTPase-activating A0A0C4DGN6 GIT1 1.306E-03 -8.810E-01 1.115E-03 -7.228E-01 protein GIT1 Methylglutaconyl-CoA Q13825 AUH 6.097E-04 -7.666E-01 5.619E-06 -1.178E+00 hydratase, mitochondrial ADP/ATP translocase 1 P12235 SLC25A4 6.068E-03 -6.095E-01 3.595E-04 -1.011E+00 MIC J3QTA6 CHCHD6 1.090E-04 -5.913E-01 2.124E-03 -5.948E-01 MIC J3QTA6 CHCHD6 1.090E-04 -5.913E-01 2.124E-03 -5.948E-01 Protein kinase C and casein Q9BY11 PACSIN1 3.837E-03 -5.863E-01 3.680E-06 -1.824E+00 kinase substrate in neurons protein 1 Tubulin polymerization- O94811 TPPP 6.466E-03 -5.755E-01 6.943E-06 -1.169E+00 promoting protein MIC C9JRZ6 CHCHD3 2.912E-02 -6.187E-01 2.195E-03 -9.781E-01 Mitochondrial 2-