Suckley's Cuckoo Bumblebee Listing Petition
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Bumble Bees of the Western United States” by Jonathan Koch, James Strange, and Paul Williams (2012)
Adapted from the “Bumble Bees of the Western United States” by Jonathan Koch, James Strange, and Paul Williams (2012). Bumble bees are one of Wyoming’s most important and mild-mannered pollinators. There are more than 20 species in Wyoming, which you can often tell apart by the color patterns on their bodies. This guide shows color patterns for queen bees only, and some species can have multiple queen Bumble patterns! of Bees Areas in yellow indicate where each species Wyoming is found in Wyoming wyomingbiodiversity.org Black Tail Bumble Bee How can you tell Bombus melanopygus it's a bumble bee? Common Bumble bees are the largest bodied bees in Wyoming. Queens can be up to two inches long, but most queens and workers are somewhat smaller than that. They're very hairy all over their bodies, and carry pollen in “baskets” on their hind legs. Did you find a bumble bee? Submit your observation to the Xerces Society of Invertebrate Conservation's website: BumbleBeeWatch.org You can be a member of the citizen science community! Brown-Belted Bumble Bee California Bumble Bee Bombus griseocollis Bombus californicus Common Uncommon Central Bumble Bee Cuckoo Bumble Bee Bombus centralis Bombus insularis Common Common Fernald Cuckoo Bumble Bee Forest Bumble Bee Bombus fernaldae Bombus sylvicola Uncommon Uncommon Frigid Bumble Bee Fuzzy-Horned Bumble Bee Bombus frigidus Bombus mixtus Rare Common Half-Black Bumble Bee High Country Bumble Bee Bombus vagans Bombus balteatus Common Common Hunt’s Bumble Bee Morrison Bumble Bee Bombus huntii Bombus morrisoni Common Common Nevada Bumble Bee Red-Belted Bumble Bee Bombus nevadensis Bombus rufocinctus Common Common Suckley Cuckoo Bumble Bee Bombus suckleyi Red-Belted Bumble Bee, continued Uncommon Two-Form Bumble Bee Western Bumble Bee Bombus bifarius Bombus occidentalis Common Rare throughout much of its range, but common in Wyoming. -

Native and Invasive Ants Affect Floral Visits of Pollinating Honey Bees in Pumpkin Flowers (Cucurbita Maxima)
www.nature.com/scientificreports OPEN Native and invasive ants afect foral visits of pollinating honey bees in pumpkin fowers (Cucurbita maxima) Anjana Pisharody Unni1,3, Sajad Hussain Mir1,2, T. P. Rajesh1, U. Prashanth Ballullaya1, Thomas Jose1 & Palatty Allesh Sinu1* Global pollinator decline is a major concern. Several factors—climate change, land-use change, the reduction of fowers, pesticide use, and invasive species—have been suggested as the reasons. Despite being a potential reason, the efect of ants on fowers received less attention. The consequences of ants being attracted to nectar sources in plants vary depending upon factors like the nectar source’s position, ants’ identity, and other mutualists interacting with the plants. We studied the interaction between fower-visiting ants and pollinators in Cucurbita maxima and compared the competition exerted by native and invasive ants on its pollinators to examine the hypothesis that the invasive ants exacerbate more interference competition to pollinators than the native ants. We assessed the pollinator’s choice, visitation rate, and time spent/visit on the fowers. Regardless of species and nativity, ants negatively infuenced all the pollinator visitation traits, such as visitation rate and duration spent on fowers. The invasive ants exerted a higher interference competition on the pollinators than the native ants did. Despite performing pollination in fowers with generalist pollination syndrome, ants can threaten plant-pollinator mutualism in specialist plants like monoecious plants. A better understanding of factors infuencing pollination will help in implementing better management practices. Humans brought together what continents isolated. One very conspicuous evidence of this claim rests in the rearranged biota. -

Classification of the Apidae (Hymenoptera)
Utah State University DigitalCommons@USU Mi Bee Lab 9-21-1990 Classification of the Apidae (Hymenoptera) Charles D. Michener University of Kansas Follow this and additional works at: https://digitalcommons.usu.edu/bee_lab_mi Part of the Entomology Commons Recommended Citation Michener, Charles D., "Classification of the Apidae (Hymenoptera)" (1990). Mi. Paper 153. https://digitalcommons.usu.edu/bee_lab_mi/153 This Article is brought to you for free and open access by the Bee Lab at DigitalCommons@USU. It has been accepted for inclusion in Mi by an authorized administrator of DigitalCommons@USU. For more information, please contact [email protected]. 4 WWvyvlrWryrXvW-WvWrW^^ I • • •_ ••^«_«).•>.• •.*.« THE UNIVERSITY OF KANSAS SCIENC5;^ULLETIN LIBRARY Vol. 54, No. 4, pp. 75-164 Sept. 21,1990 OCT 23 1990 HARVARD Classification of the Apidae^ (Hymenoptera) BY Charles D. Michener'^ Appendix: Trigona genalis Friese, a Hitherto Unplaced New Guinea Species BY Charles D. Michener and Shoichi F. Sakagami'^ CONTENTS Abstract 76 Introduction 76 Terminology and Materials 77 Analysis of Relationships among Apid Subfamilies 79 Key to the Subfamilies of Apidae 84 Subfamily Meliponinae 84 Description, 84; Larva, 85; Nest, 85; Social Behavior, 85; Distribution, 85 Relationships among Meliponine Genera 85 History, 85; Analysis, 86; Biogeography, 96; Behavior, 97; Labial palpi, 99; Wing venation, 99; Male genitalia, 102; Poison glands, 103; Chromosome numbers, 103; Convergence, 104; Classificatory questions, 104 Fossil Meliponinae 105 Meliponorytes, -

Wisconsin Bee Identification Guide
WisconsinWisconsin BeeBee IdentificationIdentification GuideGuide Developed by Patrick Liesch, Christy Stewart, and Christine Wen Honey Bee (Apis mellifera) The honey bee is perhaps our best-known pollinator. Honey bees are not native to North America and were brought over with early settlers. Honey bees are mid-sized bees (~ ½ inch long) and have brownish bodies with bands of pale hairs on the abdomen. Honey bees are unique with their social behavior, living together year-round as a colony consisting of thousands of individuals. Honey bees forage on a wide variety of plants and their colonies can be useful in agricultural settings for their pollination services. Honey bees are our only bee that produces honey, which they use as a food source for the colony during the winter months. In many cases, the honey bees you encounter may be from a local beekeeper’s hive. Occasionally, wild honey bee colonies can become established in cavities in hollow trees and similar settings. Photo by Christy Stewart Bumble bees (Bombus sp.) Bumble bees are some of our most recognizable bees. They are amongst our largest bees and can be close to 1 inch long, although many species are between ½ inch and ¾ inch long. There are ~20 species of bumble bees in Wisconsin and most have a robust, fuzzy appearance. Bumble bees tend to be very hairy and have black bodies with patches of yellow or orange depending on the species. Bumble bees are a type of social bee Bombus rufocinctus and live in small colonies consisting of dozens to a few hundred workers. Photo by Christy Stewart Their nests tend to be constructed in preexisting underground cavities, such as former chipmunk or rabbit burrows. -

Seasonal and Spatial Patterns of Mortality and Sex Ratio in the Alfalfa
Seasonal and spatial patterns of mortality and sex ratio in the alfalfa leafcutting bee, Megachile rotundata (F.) by Ruth Pettinga ONeil A thesis submitted in partial fulfillment of the requirements for the degree of Master of Science in Entomology Montana State University © Copyright by Ruth Pettinga ONeil (2004) Abstract: Nests from five seed alfalfa sites of the alfalfa leafcutting bee Megachile rotundata (F.) were monitored over the duration of the nesting season in 2000 and 2001, from early July through late August. Cells containing progeny of known age and known position within the nest were subsequently analyzed for five commonly encountered categories of pre-diapause mortality in this species. Chalkbrood and pollen ball had the strongest seasonal relationships of mortality factors studied. Chalkbrood incidence was highest in early-produced cells. Pollen ball was higher in late-season cells. Chalkbrood, parasitism by the chalcid Pteromalus venustus, and death of older larvae and prepupae , due to unknown source(s) exhibited the strongest cell-position relationships. Both chalkbrood and parasitoid incidence were highest in the inner portions of nests. The “unknown” category of mortality was highest in outer portions of nests. Sex ratio was determined for a subset of progeny reared to adulthood. The ratio of females to males is highest in cells in inner nest positions. Sex ratio is female-biased very early in the nesting season, when all cells being provisioned are the inner cells of nests, due to the strong positional effect on sex ratio. SEASONAL AND SPATIAL PATTERNS OF MORTALITY AND SEX RATIO IN THE ALFALFA LEAFCUTTING BEE, Megachile rotundata (F.) by . -
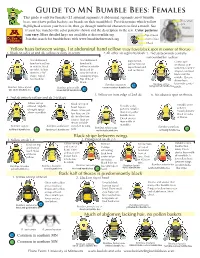
Guide to MN Bumble Bees: Females
Guide to MN Bumble Bees: Females This guide is only for females (12 antennal segments, 6 abdominal segments, most bumble Three small bees, most have pollen baskets, no beards on their mandibles). First determine which yellow eyes highlighted section your bee is in, then go through numbered characters to find a match. See if your bee matches the color patterns shown and the description in the text. Color patterns ® can vary. More detailed keys are available at discoverlife.org. Top of head Bee Front of face Squad Join the search for bumble bees with www.bumbleebeewatch.org Cheek Yellow hairs between wings, 1st abdominal band yellow (may have black spot in center of thorax) 1. Black on sides of 2nd ab, yellow or rusty in center 2.All other ab segments black 3. 2nd ab brownish centrally surrounded by yellow 2nd abdominal 2nd abdominal Light lemon Center spot band with yellow band with yellow hairs on on thorax with in middle, black yellow in middle top of head and sometimes faint V on sides. Yellow bordered by and on thorax. shaped extension often in a “W” rusty brown in a back from the shape. Top of swooping shape. middle. Queens head yellow. Top of head do not have black. Bombus impatiens Bombus affinis brownish central rusty patched bumble bee Bombus bimaculatus Bombus griseocollis common eastern bumble bee C patch. two-spotted bumble bee C brown-belted bumble bee C 5. Yellow on front edge of 2nd ab 6. No obvious spot on thorax. 4. 2nd ab entirely yellow and ab 3-6 black Yellow on top Black on top of Variable color of head. -

NIH Public Access Author Manuscript J Eukaryot Microbiol
NIH Public Access Author Manuscript J Eukaryot Microbiol. Author manuscript; available in PMC 2009 November 18. NIH-PA Author ManuscriptPublished NIH-PA Author Manuscript in final edited NIH-PA Author Manuscript form as: J Eukaryot Microbiol. 2009 ; 56(2): 142±147. doi:10.1111/j.1550-7408.2008.00374.x. Morphological, molecular, and phylogenetic characterization of Nosema ceranae, a microsporidian parasite isolated from the European honey bee, Apis mellifera Y.P. Chena, J.D. Evansa, C. Murphyb, R. Gutellc, M. Zukerd, D. Gundensen-Rindale, and J.S. Pettisa a USDA-ARS, Bee Research Laboratory, Beltsville, MD, USA b USDA-ARS, Soybean Genomic & Improvement Laboratory, Beltsville, MD, USA c Institute for Cellular and Molecular Biology and Section of Integrative Biology, University of Texas, Austin, TX, USA d Rensselaer Polytechnic Institute, NY, USA e USDA-ARS, Invasive Insect Biocontrol and Behavior Laboratory, Beltsville, MD, USA Abstract Nosema ceranae, a microsporidian parasite originally described from Apis cerana, has been found to infect Apis melllifera and is highly pathogenic to its new host. In the present study, data on the ultrastructure of N. ceranae, presence of N. ceranae-specific nucleic acid in host tissues, and phylogenetic relationships with other microsporidia species are described. The ultrastructural features indicate that N. ceranae possesses all of the characteristics of the genus Nosema. Spores of N. ceranae measured approximately 4.4 × 2.2 μm on fresh smears. The number of coils of the polar filament inside spores was 18--21. PCR signals specific for N. ceranae were detected not only in the primary infection site, the midgut, but also in the tissues of hypopharyngeal glands, salivary glands, Malpighian tubules, and fat body. -

The Conservation Management and Ecology of Northeastern North
THE CONSERVATION MANAGEMENT AND ECOLOGY OF NORTHEASTERN NORTH AMERICAN BUMBLE BEES AMANDA LICZNER A DISSERTATION SUBMITTED TO THE FACULTY OF GRADUATE STUDIES IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY GRADUATE PROGRAM IN BIOLOGY YORK UNIVERSITY TORONTO, ONTARIO September 2020 © Amanda Liczner, 2020 ii Abstract Bumble bees (Bombus spp.; Apidae) are among the pollinators most in decline globally with a main cause being habitat loss. Habitat requirements for bumble bees are poorly understood presenting a research gap. The purpose of my dissertation is to characterize the habitat of bumble bees at different spatial scales using: a systematic literature review of bumble bee nesting and overwintering habitat globally (Chapter 1); surveys of local and landcover variables for two at-risk bumble bee species (Bombus terricola, and B. pensylvanicus) in southern Ontario (Chapter 2); identification of conservation priority areas for bumble bee species in Canada (Chapter 3); and an analysis of the methodology for locating bumble bee nests using detection dogs (Chapter 4). The main findings were current literature on bumble bee nesting and overwintering habitat is limited and biased towards the United Kingdom and agricultural habitats (Ch.1). Bumble bees overwinter underground, often on shaded banks or near trees. Nests were mostly underground and found in many landscapes (Ch.1). B. terricola and B. pensylvanicus have distinct habitat characteristics (Ch.2). Landscape predictors explained more variation in the species data than local or floral resources (Ch.2). Among local variables, floral resources were consistently important throughout the season (Ch.2). Most bumble bee conservation priority areas are in western Canada, southern Ontario, southern Quebec and across the Maritimes and are most often located within woody savannas (Ch.3). -

Bumble Bees of CT-Females
Guide to CT Bumble Bees: Females This guide is only for females (12 antennal segments, 6 abdominal segments, most bumble bees, most have pollen baskets, no beards on their mandibles). First determine which yellow Three small eyes highlighted section your bee is in, then go through numbered characters to find a match. See if your bee matches the color patterns shown and the description in the text. Color patterns can vary. More detailed keys are available at discoverlife.org. Top of head Join the search for bumble bees with www.bumbleebeewatch.org Front of face Cheek Yellow hairs between wings, 1st abdominal band yellow (may have black spot in center of thorax) 1. Black on sides of 2nd ab, yellow or rusty in center 2.All other ab segments black 3. 2nd ab brownish centrally surrounded by yellow 2nd abdominal 2nd abdominal Light lemon Center spot band with yellow band with yellow hairs on on thorax with in middle, black yellow in middle top of head and sometimes faint V on sides. Yellow bordered by and on thorax. shaped extension often in a “W” rusty brown in a back from the shape. Top of swooping shape. middle. Queens head yellow. Top of head do not have black. Bombus impatiens Bombus affinis brownish central rusty patched bumble bee Bombus bimaculatus Bombus griseocollis common eastern bumble bee patch. two-spotted bumble bee brown-belted bumble bee 4. 2nd ab entirely yellow and ab 3-6 black 5. No obvious spot on thorax. Yellow on top Black on top of Variable color of head. -

Ants and Bees Date: May 13-17
Toddler Club Program Lesson Plan Theme 10: Bugs Week 2: Ants and Bees Date: May 13-17 Objective: This week the children will learn about ants and bees. The will learn how to identify these Parents as Partners: Card #38 insects and how to stay safely away from their bites and stings. Spanish Vocabulary: hormiga, hormiguero, abeja, picadura, torax, abdomen, English Vocabulary: ant, anthill, bee, sting, thorax, abdomen, antennae, bug antenas, bicho American Sign Language (ASL): ant, anthill, bee, sting, thorax, abdomen, antennae, bug LESSON Monday Tuesday Wednesday Thursday Friday COMPONENTS UNITE:" Shoo Fly." Ask children if UNITE: Sing "The Insect Song" head, UNITE: Discuss the places where we UNITE: Act out the song, "Baby UNITE: Review all that we learned they have been bothered by a fly thorax and abdomen song in the can find bugs and mosquitos. Bumblebee." about ants and bees. CALM: Learn and practice the tune of head, shoulders, knees and CALM: Point out the "bzz, bzz, bzz" CALM: Invite the children to copy CALM: Talk about breathing "buzzing breathing." toes. sound that the bees make is called this breathing pattern. exercises and how they relax you. CONNECT: Send well wishes to all CALM: Learn to take deep breath and the buzzing sound. CONNECT: Play a game with your CONNECT: Partner children and Starting the Day the kids that are absent. release it. CONNECT: Demonstrate the Index finger by making circles in the have them try this game, and then BUILD COMMUNITY: Pass around CONNECT: Use Max to welcome anticipation game called, Buzzing air with a buzzing sound and landing switch roles. -

Within Colony Dynamics of Nosema Bombi Infections: Disease Establishment, Epidemiology and Potential Vertical Transmission Samina T
Within colony dynamics of Nosema bombi infections: disease establishment, epidemiology and potential vertical transmission Samina T. Rutrecht, Mark J.F. Brown To cite this version: Samina T. Rutrecht, Mark J.F. Brown. Within colony dynamics of Nosema bombi infections: disease establishment, epidemiology and potential vertical transmission. Apidologie, Springer Verlag, 2008, 39 (5), pp.504-514. hal-00891946 HAL Id: hal-00891946 https://hal.archives-ouvertes.fr/hal-00891946 Submitted on 1 Jan 2008 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Apidologie 39 (2008) 504–514 Available online at: c INRA/DIB-AGIB/ EDP Sciences, 2008 www.apidologie.org DOI: 10.1051/apido:2008031 Original article Within colony dynamics of Nosema bombi infections: disease establishment, epidemiology and potential vertical transmission* Samina T. Rutrecht1,2,MarkJ.F.Brown1 1 Department of Zoology, School of Natural Sciences, Trinity College Dublin, Dublin 2, Ireland 2 Windward Islands Research and Education Foundation, PO Box 7, Grenada, West Indies Received 20 November 2007 – Revised 27 February 2008 – Accepted 28 March 2008 Abstract – Successful growth and transmission is a prerequisite for a parasite to maintain itself in its host population. Nosema bombi is a ubiquitous and damaging parasite of bumble bees, but little is known about its transmission and epidemiology within bumble bee colonies. -

Meet the Rare, Threatened and Endangered Insect Pollinators Of
NDSU EXTENSION E1977 Meet the Rare, Threatened and Endangered Jeremy Hemberger Johanna James-Heinze Insect Pollinators of North Dakota David Lowenstein, Consumer Horticulture Extension Educator, Michigan State University Nathaniel Walton, Consumer Horticulture Extension Educator, Michigan State University Patrick Beauzay, Integrated Pest Management Coordinator and Research Specialist, NDSU Veronica Calles-Torrez, Post-doctoral Scientist, NDSU Gerald Fauske, Insect Collection Manager and Research Specialist, NDSU David L. Cuthrell, Esther McGinnis, Extension Horticulturist, NDSU Michigan State University Janet Knodel, Extension Entomologist, NDSU Erik Runquist, Minnesota Zoo Why are some pollinators in decline? Rusty Patched Bumble Bee (Bombus affinis) and Nectar, pollen and habitat are three major requirements of Yellow-banded Bumble Bee (B. terricola) pollinators. When habitats (for example, natural areas) are lost to Gardeners frequently see and recognize bumble bees throughout agriculture, residential homes or commercial spaces, some insect the growing season, but some species have declined rapidly in the pollinators can undergo a rapid decline. Specialized pollinators are past few decades. Two declining bumble bee species are native to more susceptible to habitat or food losses because they often are the northern U.S. from the Dakotas eastward. dependent on a few specific host plants in a specialized habitat. Both feed on specific plants, compared with other bumble bees, Environmental contamination from using herbicides that which feed on a wider host plant list. In addition to habitat loss, prevent flowers from blooming or insecticides that kill pollinators their decline may be caused by a natural disadvantage in tolerating immediately or through time degrades otherwise suitable habitats. pathogens spread from commercially reared bumble bees.