View PDF Version
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
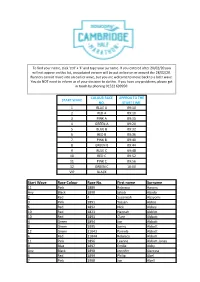
Start Wave Race Colour Race No. First Name Surname
To find your name, click 'ctrl' + 'F' and type your surname. If you entered after 20/02/20 you will not appear on this list, an updated version will be put online on or around the 28/02/20. Runners cannot move into an earlier wave, but you are welcome to move back to a later wave. You do NOT need to inform us of your decision to do this. If you have any problems, please get in touch by phoning 01522 699950. COLOUR RACE APPROX TO THE START WAVE NO. START TIME 1 BLUE A 09:10 2 RED A 09:10 3 PINK A 09:15 4 GREEN A 09:20 5 BLUE B 09:32 6 RED B 09:36 7 PINK B 09:40 8 GREEN B 09:44 9 BLUE C 09:48 10 RED C 09:52 11 PINK C 09:56 12 GREEN C 10:00 VIP BLACK Start Wave Race Colour Race No. First name Surname 11 Pink 1889 Rebecca Aarons Any Black 1890 Jakob Abada 2 Red 4 Susannah Abayomi 3 Pink 1891 Yassen Abbas 6 Red 1892 Nick Abbey 10 Red 1823 Hannah Abblitt 10 Red 1893 Clare Abbott 4 Green 1894 Jon Abbott 8 Green 1895 Jonny Abbott 12 Green 11043 Pamela Abbott 6 Red 11044 Rebecca Abbott 11 Pink 1896 Leanne Abbott-Jones 9 Blue 1897 Emilie Abby Any Black 1898 Jennifer Abecina 6 Red 1899 Philip Abel 7 Pink 1900 Jon Abell 10 Red 600 Kirsty Aberdein 6 Red 11045 Andrew Abery Any Black 1901 Erwann ABIVEN 11 Pink 1902 marie joan ablat 8 Green 1903 Teresa Ablewhite 9 Blue 1904 Ahid Abood 6 Red 1905 Alvin Abraham 9 Blue 1906 Deborah Abraham 6 Red 1907 Sophie Abraham 1 Blue 11046 Mitchell Abrams 4 Green 1908 David Abreu 11 Pink 11047 Kathleen Abuda 10 Red 11048 Annalisa Accascina 4 Green 1909 Luis Acedo 10 Red 11049 Vikas Acharya 11 Pink 11050 Catriona Ackermann -

2006 Kyoto, Japan
October 28 - November 2, 2006 ~ Kyoto, Japan ~ Final Program Table of Contents Welcome . .2 Acknowledgements . .3 Organization . .6 MDS .Committees .& Task. .Forces . .9 International .Congress .Registration .and Venue. .12 International .Congress .Information . 13-15 . Continuing .Medical .Education . .13 . Evaluations . .14 . Press .Room . .15 Program-at-a-Glance . .17 Scientific Session Definitions . .19 Scientific .Sessions . .21 Faculty . .51 Committee .& Task. .Force .Meetings . .56 Exhibitor .Information . .57 Exhibitor .Directory . .58 Floor .Plans . 62-64 Map .of .Kyoto . .66 Lunch Map . .67 Subway Map . .68 Social Events . .69 Poster .Session .1 . .72 Poster .Session .2 . .88 Poster .Session .3 . .102 Poster .Session .4 . .117 CME .Request .Form . .133 The Movement. .Disorder .Society’s 0th International Congress of Parkinson’s Disease and Movement Disorders Welcome Letter Dear Colleagues, On behalf of The Movement Disorder Society (MDS), we are pleased to welcome you to Kyoto, Japan for the 10th International Congress of Parkinson’s Disease and Movement Disorders . The 10th International Congress has been designed to provide an innovative and comprehensive overview of the latest perspectives and research developments in the field of Movement Disorders . We encourage you to take every opportunity to participate in the Scientific Program which has drawn world renowned speakers and foremost experts in their respective fields . In the next days, the latest research regarding Movement Disorders will be presented and discussed in an open format, offering unique educational opportunities for all delegates . The International Congress convenes with a series of Opening Seminars and then continues with an array of Plenary, Parallel, Poster and Video Sessions, as well as Lunch Seminars, Controversies and Skills Workshops . -

Aes Corporation
THE AES CORPORATION THE AES CORPORATION The global power company A Passion to Serve A Passion A PASSION to SERVE 2000 ANNUAL REPORT ANNUAL REPORT THE AES CORPORATION 1001 North 19th Street 2000 Arlington, Virginia 22209 USA (703) 522-1315 CONTENTS OFFICES 1 AES at a Glance AES CORPORATION AES HORIZONS THINK AES (CORPORATE OFFICE) Richmond, United Kingdom Arlington, Virginia 2 Note from the Chairman 1001 North 19th Street AES OASIS AES TRANSPOWER Arlington, Virginia 22209 Suite 802, 8th Floor #16-05 Six Battery Road 5 Our Annual Letter USA City Tower 2 049909 Singapore Phone: (703) 522-1315 Sheikh Zayed Road Phone: 65-533-0515 17 AES Worldwide Overview Fax: (703) 528-4510 P.O. Box 62843 Fax: 65-535-7287 AES AMERICAS Dubai, United Arab Emirates 33 AES People Arlington, Virginia Phone: 97-14-332-9699 REGISTRAR AND Fax: 97-14-332-6787 TRANSFER AGENT: 83 2000 AES Financial Review AES ANDES FIRST CHICAGO TRUST AES ORIENT Avenida del Libertador COMPANY OF NEW YORK, 26/F. Entertainment Building 602 13th Floor A DIVISION OF EQUISERVE 30 Queen’s Road Central 1001 Capital Federal P.O. Box 2500 Hong Kong Buenos Aires, Argentina Jersey City, New Jersey 07303 Phone: 852-2842-5111 Phone: 54-11-4816-1502 USA Fax: 852-2530-1673 Fax: 54-11-4816-6605 Shareholder Relations AES AURORA AES PACIFIC Phone: (800) 519-3111 100 Pine Street Arlington, Virginia STOCK LISTING: Suite 3300 NYSE Symbol: AES AES ENTERPRISE San Francisco, California 94111 Investor Relations Contact: Arlington, Virginia USA $217 $31 Kenneth R. Woodcock 93% 92% AES ELECTRIC Phone: (415) 395-7899 $1.46* 91% Senior Vice President 89% Burleigh House Fax: (415) 395-7891 88% 1001 North 19th Street $.96* 18 Parkshot $.84* AES SÃO PAULO Arlington, Virginia 22209 Richmond TW9 2RG $21 Av. -
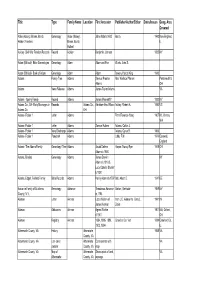
Vertical File
Title Type Family Name Location First Ancestor Publisher/Author/Editor Dates/Issues Geog. Area Covered Abbe (Abbey), Brown, Burch, Genealogy Abbe (Abbey), John Abbe b.1613 Burch 1943 New England, Hulbert Families Brown, Burch, IL Hulbert Ackley- Civil War Pension Records Record Ackley Benjamin Johnson 1832 NY Adam (Biblical)- Bible Genealogies Genealogy Adam Adam and Eve Wurts, John S. Adam (Biblical)- Book of Adam Genealogy Adam Adam Bowen, Harold King 1943 Adams Family Tree Adams Samuel Preston Mrs. Wallace Phorsm Portsmouth & Adams OH Adams News Release Adams James Taylor Adams VA Adams - Spring Family Record Adams James Wamorth? 1832 NY Adams Co., OH- Early Marriages in Records Adams Co., Abraham thru Wilson Ackley, Robert A. 1982 US Adams Co. OH Adams- Folder 1 Letter Adams From Florence Hoag 1907 Mt. Vernon, WA Adams- Folder 1 Letter Adams Samuel Adams Adams, Calvin J. Adams- Folder 1 Navy Discharge Adams Adams, Cyrus B. 1866 Adams- Folder 1 Pamphlet Adams Lobb, F.M. 1979 Cornwall, England Adams- The Adams Family Genealogy/ Tree Adams Jacob Delmar Harper, Nancy Eyer 1978 OH Adams b.1858 Adams, Broyles Genealogy Adams James Darwin KY Adams b.1818 & Lucy Ophelia Snyder b.1820 Adams, Edgell, Twiford Family Bible Records Adams Henry Adams b.1797 Bell, Albert D. 1947 DE Adriance Family of Dutchess Genealogy Adriance Theodorus Adriance Barber, Gertrude 1959 NY County, N.Y. m.1783 Aikman Letter Aikman Lists children of from L.C. Aikman to Cora L. 1941 IN James Aikman Davis Aikman Obituaries Aikman Agnes Ritchie 1901 MA, Oxford, d.1901 OH Aikman Registry Aikman 1884, 1895, 1896, Crawford Co. -

1 CURRICULUM VITAE NAME: Michael Parkin DATE of BIRTH
CURRICULUM VITAE (October 2011) NAME: Michael Parkin DATE OF BIRTH: February 21, 1939 MARITAL STATUS: Married: Dr. Robin Bade Children: Catherine (1964), Richard (1965), Ann (1967) CITIZENSHIP: Canadian, British, Australian HOME ADDRESS: 128 Bloomfield Drive, London, Ontario, Canada, N6G 1P3 Telephone: 519-657-2622 Facsimile: 519-657-4751 BUSINESS ADDRESS: Department of Economics, University of Western Ontario, London, Ontario, Canada, N6A 5C2 Telephone: 519-661-3500 Facsimile: 519-661-3666 EDUCATION: 1952-55 Barnsley Secondary Technical School 1955-60 Correspondence courses 1960-63 University of Leicester DEGREES HELD: 1960 Associate of Institute of Management Accountants AIMA 1963 B.A. (Leicester), First class honors 1970 M.A. Economics (Manchester) 2010 D. Litt. (Leicester), Honorary APPOINTMENTS: 1955-1960 Cost accountancy in steel industry 1963-1964 Assistant Lecturer, University of Sheffield 1964-1966 Lecturer, University of Leicester 1967-1969 Lecturer, University of Essex 1969-1970 Senior Lecturer, University of Essex 1970-1975 Professor, University of Manchester 1975-2004 Professor, University of Western Ontario 2004- Professor Emeritus, University of Western Ontario 1 VISITING APPOINTMENTS: 1972 Visiting Professor, Brown University 1973 Visiting Professor, University of New South Wales 1973 Visiting Senior Research Economist, Reserve Bank of Australia 1974 Visiting Professor, Osmania University, Hyderabad, India 1979-1980 Visiting Fellow, Hoover Institution, Stanford University 1983 Visiting Scholar from Abroad, Bank of Japan, Tokyo 1991 Visiting Professor, Bond University, Australia 1992-1993 Norwich Australia Visiting Professor, Bond University, Australia 1993 Erskine Visiting Fellow, Canterbury University, Christchurch, New Zealand 1994 Visiting Professor, Bond University, Australia 1995 Visiting Professor, Bond University, Australia RESEARCH GRANTS AND AWARDS: 1968-1970 The Portfolio Behaviour of the U.K. -

NOSTALGIA, EMOTIONALITY, and ETHNO-REGIONALISM in PONTIC PARAKATHI SINGING by IOANNIS TSEKOURAS DISSERTATION Submitted in Parti
NOSTALGIA, EMOTIONALITY, AND ETHNO-REGIONALISM IN PONTIC PARAKATHI SINGING BY IOANNIS TSEKOURAS DISSERTATION Submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy in Musicology in the Graduate College of the University of Illinois at Urbana-Champaign, 2016 Urbana, Illinois Doctoral Committee: Associate Professor Donna A. Buchanan, Chair Professor Emeritus Thomas Turino Professor Gabriel Solis Professor Maria Todorova ABSTRACT This dissertation explores the multilayered connections between music, emotionality, social and cultural belonging, collective memory, and identity discourse. The ethnographic case study for the examination of all these relations and aspects is the Pontic muhabeti or parakathi. Parakathi refers to a practice of socialization and music making that is designated insider Pontic Greek. It concerns primarily Pontic Greeks or Pontians, the descendants of the 1922 refugees from Black Sea Turkey (Gr. Pontos), and their identity discourse of ethno-regionalism. Parakathi references nightlong sessions of friendly socialization, social drinking, and dialogical participatory singing that take place informally in coffee houses, taverns, and households. Parakathi performances are reputed for their strong Pontic aesthetics, traditional character, rich and aesthetically refined repertoire, and intense emotionality. Singing in parakathi performances emerges spontaneously from verbal socialization and emotional saturation. Singing is described as a confessional expression of deeply personal feelings -

Michigan State University Commencement Spring 2021
COMMENCEMENT CEREMONIES SPRING 2021 “Go forth with Spartan pride and confdence, and never lose the love for learning and the drive to make a diference that brought you to MSU.” Samuel L. Stanley Jr., M.D. President Michigan State University Photo above: an MSU entrance marker of brick and limestone, displaying our proud history as the nation’s pioneer land-grant university. On this—and other markers—is a band of alternating samara and acorns derived from maple and oak trees commonly found on campus. This pattern is repeated on the University Mace (see page 13). Inside Cover: Pattern of alternating samara and acorns. Michigan State University photos provided by University Communications. ENVIRONMENTAL TABLE OF CONTENTS STEWARDSHIP Mock Diplomas and the COMMENCEMENT Commencement Program Booklet 3-5 Commencement Ceremonies Commencement mock diplomas, 6 The Michigan State University Board of Trustees which are presented to degree 7 Michigan State University Mission Statement candidates at their commencement 8–10 Congratulatory Letters from the President, Provost, and Executive Vice President ceremonies, are 30% post-consumer 11 Michigan State University recycled content. The Commencement 12 Ceremony Lyrics program booklet is 100% post- 13 University Mace consumer recycled content. 14 Academic Attire Caps and Gowns BACCALAUREATE DEGREES Graduating seniors’ caps and gowns 16 Honors and master’s degrees’ caps and 17-20 College of Agriculture and Natural Resources gowns are made of post-consumer 21-22 Residential College in the Arts and Humanities recycled content; each cap and 23-25 College of Arts and Letters gown is made of a minimum of 26-34 The Eli Broad College of Business 23 plastic bottles. -

Degree Congregations December 2010 the Inauguration of the University of Manchester
The University of Manchester Degree Congregations December 2010 The Inauguration of The University of Manchester At midnight on 30 September 2004, UMIST and the Victoria University of Manchester were dissolved and the single institution of The University of Manchester came into existence. The formal Inauguration of the University took place on Friday, 22 October 2004 when Her Majesty The Queen visited the campus to personally present the University’s new Royal Charter to Co-Chancellor Anna Ford at a special ceremony held in the Whitworth Hall. The new institution has a distinguished heritage. It can trace its roots back to the formation of the Manchester Mechanics Institute in 1824, which later became UMIST. The Victoria University of Manchester was founded as Owens College in 1851 and became England’s first civic university in 1880. The two universities first began working together almost 100 years ago. They were situated on neighbouring campuses and this enabled them to develop a number of joint courses, departments and services. They took the decision to merge in 2003. The creation of The University of Manchester has been hailed as one of the boldest and most ambitious initiatives in higher education. This pioneering spirit carries on today, with the aim to become one of the world’s top 25 universities by 2015 backed by an ongoing £650 million investment programme in staff and facilities – the largest such investment programme in British higher education. From the President and Vice-Chancellor It is with immense pleasure that one of my first duties as the new President and Vice-Chancellor of our great University is to welcome you all – graduands, family members and friends – to The University of Manchester for this degree congregation. -

Most Common Surnames
Most Common Surnames Surnames occurring most often in Scotland's registers of Births, Marriages and Deaths Counting only the surname of the child for births, the surnames of both bride and groom for marriages, and the surname of the deceased for deaths Note: the surnames from these registers may not be representative of the surnames of the population of Scotland as a whole, as (a) they include the surnames of non-residents who were born / married / died here; (b) they exclude the surnames of residents who were born / married / died elsewhere; and (c) some age-groups have very low birth, marriage and death rates; others account for most births, marriages and deaths. Registration Year=2015 Position Surname Number 1 SMITH 1929 2 BROWN 1438 3 WILSON 1352 4 STEWART 1186 5 THOMSON 1172 6 ROBERTSON 1158 7 CAMPBELL 1130 8 ANDERSON 1068 9 MURRAY 832 10 MACDONALD 823 11 TAYLOR 794 12 SCOTT 779 13 REID 771 14 CLARK 746 15 YOUNG 639 16 MORRISON 623 17 WALKER 619 18 ROSS 614 19 WATSON 595 20 GRAHAM 571 21 MITCHELL 566 22 FRASER 564 23 PATERSON 557 24 MILLER 555 25 HENDERSON 540 26 MARTIN 539 27 CAMERON 533 28 MCDONALD 531 29 HAMILTON 530 30= DAVIDSON 519 30= GRAY 519 32 DUNCAN 512 33= JOHNSTON 501 33= KERR 501 33= SIMPSON 501 36 HUNTER 477 37 BELL 474 38 FERGUSON 465 39 KELLY 459 40 ALLAN 442 41 GRANT 429 42 MACLEOD 425 43 MCLEAN 411 44 BLACK 404 45 MACKAY 402 46 WRIGHT 393 47 MACKENZIE 385 48 GIBSON 382 49 MARSHALL 379 50 KENNEDY 377 © Crown Copyright 2016 Most Common Surnames (continued) 51 WALLACE 371 52 JONES 368 53 RUSSELL 358 54 SUTHERLAND 351 -
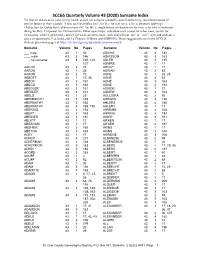
Surname Index to Find All Instances of Your Family Name, Search for Variants Caused by Poor Handwriting, Misinterpretation of Similar Letters Or Their Sounds
SCCGS Quarterly Volume 43 (2020) Surname Index To find all instances of your family name, search for variants caused by poor handwriting, misinterpretation of similar letters or their sounds. A few such examples are L for S, c for e, n for u, u for a; phonetic spellings (Aubuchon for Oubuchon); abbreviations (M’ for Mc ); single letters for double (m for mm, n for nn); translations (King for Roy, Carpenter for Zimmermann). Other search tips: substitute each vowel for other ones, search for nicknames, when hyphenated – search for each surname alone, with and without “de” or “von”; with and without a space or apostrophe (Lachance and La Chance, O’Brien and OBRIEN). More suggestions are on the SCCGS website Quarterly pages at https://stclair-ilgs.org/quarterly-surname-index/ Surname Volume No Pages Surname Volume No Pages ___ male 43 1 50 ADKINS 43 4 182 ___, male 43 3 146 ADKISSON 43 3 163 ___, no surname 43 3 120, 123, ADLER 43 3 135 127 ADMIRE 43 1 17 AACOX 43 2 74 ADOLF 43 1 17 AARON 43 1 25 ADRIAN 43 2 83 AARON 43 2 72 AGNE 43 1 24, 25 ABBOTT 43 1 17, 36 AGNE 43 2 62 ABECK 43 3 161 AGNE 43 3 163 ABEGG 43 3 163 AGNE 43 4 184 ABEGLER 43 3 161 AGNEW 43 1 31 ABEKLER 43 3 161 AGNEW 43 3 163 ABELS 43 1 25 AGULIERA 43 2 93 ABENDROTH 43 3 163 AHEARN 43 3 136 ABERNATHY 43 3 163 AHLERS 43 4 180 ABERNATHY 43 4 185, 190 AHLERT 43 1 17 ABERSOL 43 3 158 AHMANN 43 2 108 ABERT 43 3 163 AHRING 43 4 184 ABIGNER 43 3 161 AIGRE 43 3 161 ABLETT 43 1 17 AITKEN 43 1 17 ABRAHAM 43 1 17 AITKEN 43 4 197 ABSHIER 43 3 169 AKINS 43 1 17 ABSTON 43 3 163 AKINS 43 -

Mitochondrial Biogenesis and Dynamics in the Developing and Diseased Heart
Downloaded from genesdev.cshlp.org on September 27, 2021 - Published by Cold Spring Harbor Laboratory Press REVIEW Mitochondrial biogenesis and dynamics in the developing and diseased heart Gerald W. Dorn II,1 Rick B. Vega,2 and Daniel P. Kelly2 1Center for Pharmacogenomics, Washington University in St. Louis, St. Louis, Missouri 63110, USA; 2Cardiovascular Metabolism Program, Sanford Burnham Prebys Medical Discovery Institute, Orlando, Florida 32827, USA The mitochondrion is a complex organelle that serves es- fer of high-energy phosphates between the mitochondria sential roles in energy transduction, ATP production, and and the contractile apparatus. The resultant mature mito- a myriad of cellular signaling events. A finely tuned regu- chondrial system is capable of high-capacity oxidation of latory network orchestrates the biogenesis, maintenance, fuels such as fatty acid, the predominant fuel substrate and turnover of mitochondria. The high-capacity mito- for the adult heart. This review focuses on current knowl- chondrial system in the heart is regulated in a dynamic edge of the regulatory circuitry and mechanisms involved way to generate and consume enormous amounts of in the maturation and maintenance of this specialized ATP in order to support the constant pumping function high-capacity mitochondrial system in developing and in the context of changing energy demands. This review adult mammalian hearts. describes the regulatory circuitry and downstream events involved in mitochondrial biogenesis and its coordination with mitochondrial dynamics in developing and diseased Mitochondrial biogenesis: circuitry and regulatory hearts. mechanisms Mitochondrial genomic and energy transduction The adult human heart generates and consumes kilogram machinery quantities of ATP daily to support normal pump function. -
![Compiled Nell ] Parkin](https://docslib.b-cdn.net/cover/1158/compiled-nell-parkin-6191158.webp)
Compiled Nell ] Parkin
MY PRAYER IN MY SEARCH FOR MY ANC ESTERS THAT I WILL HAVE AN INQUIRING MIND A WILL TO WORK, LONG AND HARD THE STRENGTH TO PERSEVERE IN THE FACE OF DISCOURAGEMENT AND GIVE "THANKS TO THEE, THAT I MIGHT HELP THEM ETERNALLY COMPILED Nell B. P. Bair NELL ] PARKIN Compiled By This is a thought that I learned many years ago, and I have tried even though Nell B. Parkin, Bair THOUGHT FLOWERS I have been ill many times and had to spend months in bed, I have thought of these times as opportunities, and a blessing, for during The heart is the garden these times, my mind has been clear and I have Where thought flowers grow, The thought that you think kept a clip board and pencil handy, and have written many of these histories while in pain, Are the seeds that you sew. and the accomplishing of the poems and histories have helped to keep my mind off of the pain, and Each kind loving thought Bears a kind loving deed. at the close of each day, I have felt that the day wasn!t lost, for now these testimonies, mingled While a thought that is with my determination and great testimonies, that Selfish just like a weed. God lives and answers prayers, and heals our bodies if we but give Him the credit, I feel that I have lived We must watch what we think and known my ancesters, of whom I am prowd, and The live-long day and pull pray that they will be prowd of me.