Brewing with Fractionated Barley
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Brewing Grains What Is Malt?
612.724.4514 [email protected] www.aperfectpint.net Brewing Grains Brewing grains are the heart and soul of beer. Next to water they make up the bulk of brewing ingredients. Brewing grains provide the sugars that yeast ferment. They are the primary source of beer color and a major contributor to beer flavor, aroma, and body. Proteins in the grains give structure to beer foam and minerals deliver many of the nutrients essential to yeast growth. By far the most common brewing grain is malted barley or barley malt, but a variety of other grains, both malted and unmalted, are also used including wheat, corn, rice, rye, and oats. What is Malt? To put it plainly, malt is cereal grain that has undergone the malting process. In the simplest terms, malting is the controlled germination and kilning of grain. Malting develops the diastatic enzymes that accomplish the conversion of starch to sugar during brewing and begins a limited process of conversion that makes the starches more accessible to the brewer. Malting also gives brewing grains their distinctive colors and flavors. Only the highest quality grain, called brewing grade, is selected for malting. Brewing grade grain is selected for, among other things, high starch content, uniform kernel size, low nitrogen content, and high diastatic power. Diastatic power is the ability of grains to break down complex starch molecules into simpler sugars for brewing. It is determined by the amount of diastatic enzymes in the grain. Barley is the most commonly malted grain, but other grains like wheat and rye are also malted. -
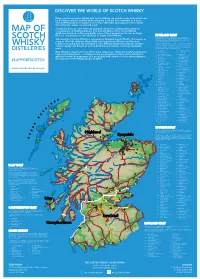
2019 Scotch Whisky
©2019 scotch whisky association DISCOVER THE WORLD OF SCOTCH WHISKY Many countries produce whisky, but Scotch Whisky can only be made in Scotland and by definition must be distilled and matured in Scotland for a minimum of 3 years. Scotch Whisky has been made for more than 500 years and uses just a few natural raw materials - water, cereals and yeast. Scotland is home to over 130 malt and grain distilleries, making it the greatest MAP OF concentration of whisky producers in the world. Many of the Scotch Whisky distilleries featured on this map bottle some of their production for sale as Single Malt (i.e. the product of one distillery) or Single Grain Whisky. HIGHLAND MALT The Highland region is geographically the largest Scotch Whisky SCOTCH producing region. The rugged landscape, changeable climate and, in The majority of Scotch Whisky is consumed as Blended Scotch Whisky. This means as some cases, coastal locations are reflected in the character of its many as 60 of the different Single Malt and Single Grain Whiskies are blended whiskies, which embrace wide variations. As a group, Highland whiskies are rounded, robust and dry in character together, ensuring that the individual Scotch Whiskies harmonise with one another with a hint of smokiness/peatiness. Those near the sea carry a salty WHISKY and the quality and flavour of each individual blend remains consistent down the tang; in the far north the whiskies are notably heathery and slightly spicy in character; while in the more sheltered east and middle of the DISTILLERIES years. region, the whiskies have a more fruity character. -

The Whiskey Machine: Nanofactory-Based Replication of Fine Spirits and Other Alcohol-Based Beverages
The Whiskey Machine: Nanofactory-Based Replication of Fine Spirits and Other Alcohol-Based Beverages © 2016 Robert A. Freitas Jr. All Rights Reserved. Abstract. Specialized nanofactories will be able to manufacture specific products or classes of products very efficiently and inexpensively. This paper is the first serious scaling study of a nanofactory designed for the manufacture of a specific food product, in this case high-value-per- liter alcoholic beverages. The analysis indicates that a 6-kg desktop appliance called the Fine Spirits Synthesizer, aka. the “Whiskey Machine,” consuming 300 W of power for all atomically precise mechanosynthesis operations, along with a commercially available 59-kg 900 W cryogenic refrigerator, could produce one 750 ml bottle per hour of any fine spirit beverage for which the molecular recipe is precisely known at a manufacturing cost of about $0.36 per bottle, assuming no reduction in the current $0.07/kWh cost for industrial electricity. The appliance’s carbon footprint is a minuscule 0.3 gm CO2 emitted per bottle, more than 1000 times smaller than the 460 gm CO2 per bottle carbon footprint of conventional distillery operations today. The same desktop appliance can intake a tiny physical sample of any fine spirit beverage and produce a complete molecular recipe for that product in ~17 minutes of run time, consuming <25 W of power, at negligible additional cost. Cite as: Robert A. Freitas Jr., “The Whiskey Machine: Nanofactory-Based Replication of Fine Spirits and Other Alcohol-Based Beverages,” IMM Report No. 47, May 2016; http://www.imm.org/Reports/rep047.pdf. 2 Table of Contents 1. -

How to Malt Barley (Or Wheat) for Beer (Or Whisky/Whiskey) I
How to Malt Barley (or Wheat) for Beer (or Whisky/Whiskey) I. Start with Good Quality Barley (or Wheat) A. When purchasing grain for malting, first ask about what types of chemicals might have been used on the grain and then about the grain's history: when it was harvested, how it was stored, etc. B. Inspect the Grain 1. Bits of chaff – and even the occasional dead bug – are quite normal and are of little concern. This sort of debris can easily be removed later when the grain is washed. It will usually float to the surface of the water where it can be skimmed off in a matter of minutes. 2. Grain that has live bugs should generally be avoided, as such bugs will spread to other grain stores and feast on all available grain until it is ready for use. Some of those bugs will also contribute a strong off-smell and off-taste, as well. However, buggy grain can occasionally be had for free, and there are a few types of bugs that do minimal damage to the grain if they are not allowed much time to do their worst. But definitely proceed cautiously with live bugs. 3. The presence of foreign seeds mixed in with the grain may or may not be problematic. A few kernels of corn, a bean or two, a few oats, etc., should not be cause for concern, but if there are unknown seeds mixed in with the grain, ask what they are and act accordingly. 4. Examine the color of the grain and avoid any that has a grayish hue, as this would indicate that the grain was mildewed, either in the field or in storage. -

Undestanding Malting Barley Quality Aaron Macleod, Director, Center for Craft Food and Beverage Hartwick College, Oneonta, NY, 13820 [email protected]
Undestanding Malting Barley Quality Aaron MacLeod, Director, Center for Craft Food and Beverage Hartwick College, Oneonta, NY, 13820 [email protected] Malt is the key ingredient in beer that provides the starch and enzymes necessary to produce the fermentable sugars which yeast then turn into alcohol. Malt also provides the color and flavor compounds which contribute to the final character of beer. Malting is the biological process that turns barley into malt. It is a three stage process including soaking (or steeping) the grain in water to bring the kernels to 45% moisture; germination under cool, humid conditions and drying (or kilning) to dry and stabilize the final malt. Barley must meet strict quality criteria to be acceptable for malt production. Maintaining tight controls on these quality factors in the grain is necessary to ensure good processing efficiency and final product quality in the malthouse and brewery. Care must be taken when growing malting barley to ensure that it meets the necessary specifications. A premium is paid to growers for high quality malting barley to compensate for the extra effort. High quality malting barley should have the following characteristics: Pure lot of an acceptable variety Germination of 95% or higher Protein content raging between 9.5% to 12.5% (dry basis) Moisture content below 13.5% Plump and uniform kernels Free of disease and low DON content Less than 5% of peeled, broken, or damaged kernels Clean and free of insects, admixtures, ergot or foreign material Varietal purity Malting barley varieties are bred specifically for characteristics that promote good malting and brewing performance, such as high enzymatic activity, as well as good agronomic performance and disease resistance. -

Breakdown of a Malt COA
Breakdown of a Malt COA Bucket Analysis Approach Presenters Tyler Schoales Mike Heinrich Craft Malt Specialist – NA Craft Sales Manager Country Malt Group Great Western Malting Breakdown of a Malt COA Agenda Overview of Malting and Modification Certificate of Analysis Breakdown Bucketing Analysis • Protein Dependent Specifications • Carbohydrate Dependent Specifications • Enzyme Package Specifications • Color Breakdown of a Malt COA Malting and Breakdown of Bucketing Modification COA Analysis Malting and the Certificate of Analysis • Malting is the controlled germination and kilning of a seed to produce the desirable brewing characteristics • Maltsters create ideal growing conditions for barley to germinate and drive modification • “Modification” is the biochemical breakdown of cell wall structures, and protein matrices in order to gain access to the starch reserves held within the endosperm • A malt Certificate of Analysis (COA) lists the results from a suite of standardized tests that serve to indicate how the malt will perform. Breakdown of a Malt COA Malting and Breakdown of Bucketing Modification COA Analysis Breakdown of the COA Malt Sieve Analysis (Assortment) • Plump kernels provide more extract than thinner kernels • Roller mill gaps are set according to the mean kernel size • A broad distribution can make mill setting difficult → poor extract recovery in the brewery • Typical analysis – 7/64 + 6/64’s (PLUMP’s) > 90% • Consistency is the key Malt Sieve Analysis (Breakage) • Damaged husks will form a poor filter bed • Fines formed -

Scotch Whisky Cereals Technical Note
- Scotch Whisky Cereals Technical Note 3rd Edition: August 2018 Scotch Whisky Cereals Technical Note Published by: The Scotch Whisky Association, Quartermile Two 2 Lister Square Edinburgh EH3 9GL Tel: 0131-222 9200 (Switchboard) E-mail: [email protected] 1st edition: January 2017 2nd edition: August 2017 3rd edition: August 2018 The text of the guidance has been drawn up by the Scotch Whisky Association and its Cereals Committee. Disclaimer This Technical Note has been provided as a document for information purposes only. It is aimed at those wishing to increase their knowledge of Scotch Whisky cereals. Decisions on procurement and the specification of cereals are ultimately a commercial matter for individual companies. The information contained in the guidance is correct at the time of going to print. © THE SCOTCH WHISKY ASSOCIATION 1 Scotch Whisky Cereals Technical Note Page CONTENTS Executive Summary 3 Introduction 3 Raw materials 3 Environmental sustainability 5 Assurance schemes 6 Commercial issues 7 Barley 9 Pot still distilling malt 9 Moisture content 11 Grain size, screenings and admixture 12 Grain damage 12 Viability 12 Nitrogen 12 Attributes for malt distilling barley 12 Grain distilling malt 14 Attributes for grain distilling barley 16 Development of new barley varieties 16 Research 16 Barley selection & testing 17 Spring barley varieties suitable for distilling 21 Wheat 23 Wheat varieties suitable for distilling 25 Attributes for distilling wheat varieties 29 Maize 30 Appendix 1 – General Scotch Whisky facts 31 Appendix 2 – Sources of information 33 Appendix 3 – Glossary of terms 35 2 Scotch Whisky Cereals Technical Note EXECUTIVE SUMMARY The Scotch Whisky Association’s (SWA) membership accounts for around 90% of the sector and aims to create conditions for long-term growth worldwide and secure Scotch’s place as the leading high-quality spirit drink. -

Thesis Total
UCC Library and UCC researchers have made this item openly available. Please let us know how this has helped you. Thanks! Title Development of a toolbox for the reduction of hordeins in barley malt beers Author(s) Taylor, Joshua P. Publication date 2016 Original citation Taylor, J. P. 2016. Development of a toolbox for the reduction of hordeins in barley malt beers. PhD Thesis, University College Cork. Type of publication Doctoral thesis Rights © 2016, Joshua P. Taylor. http://creativecommons.org/licenses/by-nc-nd/3.0/ Item downloaded http://hdl.handle.net/10468/3241 from Downloaded on 2021-10-04T08:07:22Z Ollscoil na hÉireann THE NATIONAL UNIVERSITY OF IRELAND Coláiste na hOllscoile, Corcaigh UNIVERSITY COLLEGE CORK SCHOOL OF FOOD AND NUTRITIONAL SCIENCES DEVELOPMENT OF A TOOLBOX FOR THE REDUCTION OF HORDEINS IN BARLEY MALT BEERS Thesis presented by Joshua P. Taylor BSc (Hons) Biotechnology Under the supervision of Prof. DSc. Dr. Elke K. Arendt For the degree of Doctor of Philosophy (PhD in Food Science and Technology) Head of School - Prof. Yrjö Roos January 2016 ii Table of Contents Table of Contents ......................................................................................... iii Abstract ......................................................................................................... 1 Acknowledgements ....................................................................................... 3 Declaration .................................................................................................... 4 Chapter -

WHEAT MALT in BREWING Viking Malt Malt Types
WHEAT MALT IN BREWING Viking malt malt types • pale brewing malts • dark brewing malts • pale caramel malts • dark caramel malts • roasted products • other malts like wheat malt Wheat as a raw material • wheat has no husk • requirements for malting wheat are same as for barley • two kinds of wheat available • A- type used mainly for baking • B- type used mainly for feed • brewing varieties do not exist at the moment • malting process does not differ much from pilsner malt production • faster water uptake in steep • germination in cool conditions • final kilning temperature 80 – 85 °C Production of wheat malt Barley / Wheat / Rye Steeping Germination Saccharification Roasting Roasting Kilning Pale Dark Roasted malt Caramel brewing brewing and barley malts malts malts Wheat vs barley Barley Wheat Unit Protein 11 12 % dm Fat 3 3 % dm Minerals 2,9 1,8 % dm Starch 63 64 % dm Beta-glucans 3,5 0,3 % dm Pentosans 9 8,5 % dm Source: EBC Manual of Good Practice, Malting Technology Wheat wort • Example of high gravity wort results: Analysis unit 100 % PM 20 % Wheat malt 40 % Wheat malt Extract m-% 16,19 16,29 16,23 Yield % / dm. 81,7 83,6 84,6 Soluble N mg / 100 g 815 836 853 pH 5,6 5,6 5,7 Colour °EBC 7,5 8,0 9,0 Haze F.U. EBC 5,2 8,5 10,8 ß-glucans mg / l 150 116 105 FAN mg / l 334 302 258 App. degree of ferm. % 85,9 84,6 83,8 Büchner-filtrate 15 min g 94,9 89,1 82,1 Saccharification min / °C 5/72 5/72 5/72 Wheat wort • Example of high gravity wort sugar compositions: fructose (% glucose maltose (% maltotriose fermentable dm) (% dm) dm) (% dm) -

BEST Distillers Malts for the Best Spirits
BEST Distillers Malts For The Best Spirits 1 WALLERTHEIM PRODUCTION/LOGISTICS KREIMBACH-KAULBACH PRODUCTION HEIDELBERG HEADQUARTERS 2 BESTMALZ PROFILE Palatia Malz GmbH is a traditional German Careful moisture control, sufficient time for family business that sells its products under germination, slow kilning and extremely the “BESTMALZ” brand in Germany and gentle roasting are features of state-of-the- abroad. The company was established as a art, modern malt production at BESTMALZ. flour mill in 1899 and converted into a malt house in 1904. Ever since, the family-owned RELIABLE AND SUSTAINABLE malting business has produced top-grade PRODUCTION products from barley, wheat and other We monitor our certified production process- grains that have gained recognition and es and pursue resource-efficient energy and respect in the company’s markets at home environmental management. We continu- and abroad. ously enhance our process-related quality assurance and our in-house documentation LOCATED IN THE MIDST OF NATURE systems as a matter of course. The company’s main production facility in Kreimbach-Kaulbach near Kaiserslautern OUR PHILOSOPHY was joined in the 1980s by a second malt As a mid-sized, family-owned company, house in Wallertheim near Mainz. The com- we take a long-term view to our business pany has its headquarters in Heidelberg. Set operations and planning, reinvesting a large at the heart of the best regions for cultivat- portion of our profits back into the compa- ing barley in Germany, the two production ny and conducting research and product plants currently process almost 90,000 tons development systematically as part of the of barley and other grains. -

Management of Malting for Flavor Development and Its Impact on Malt Analyses
Management of Malting for Flavor Development and its Impact on Malt Analyses Barley Improvement Conference January 12, 2015 Paul Kramer – Rahr Malting Co. • Introduction to Malting • Malt Flavor Development during Kilning • Malt Analyses Interactions • Rahr Wort Flavor Observations • Malting Process Barley Unloading Clean & Grade Transfer to Silo Kilning Germination Steeping Transfer to Silo Malt Cleaning Loading Malt Malting Process - Flavor CCP Barley Unloading Clean & Grade Transfer to Silo Kilning Germination Steeping Transfer to Silo Malt Cleaning Loading Malt Barley Conversion to Malt Products from Malting Process Malt 82% Respiration 2% Water Vapor 8% Malt Sprouts 4% Barley Byproducts 4% Barley Cleaning and Grading • Segregate barley by variety and crop year • Aspiration • Scalping • Metal removal • Gravity Steeping • Steeping is the most critical stage of the malting process • Objectives of steeping – Clean and rinse barley surface – Remove growth inhibitors – Uniformly hydrate the barley kernels – Provide O2 and remove CO2 – Control temperature to ensure uniform respiration – Hydrate barley to targeted moisture • Steeping Control Parameters – Time – Water volume immersions/sprays – Water temperature – O2 injection during immersion – CO2 removal during drain periods Germination Control Parameters • Germination Time – Variety & customer dependant – Typically 3.0 -5 days • Germination Bed Temperature – Air on or house temperature • Typically 54-600F – 100% humidity – Air off or exhaust temperature • Typically 68-720F • Airflow – -

{DOWNLOAD} the Brewing of Beer : Malting
THE BREWING OF BEER : MALTING PDF, EPUB, EBOOK Various | 64 pages | 08 Feb 2011 | Read Books | 9781446534014 | English | Alcester, United Kingdom The Brewing of Beer : Malting PDF Book When the English taxed beer according to the amount of malt it contained, the Irish got around it by using roasted raw barley for flavor. The darker the malt, the higher the Lovibond rating. These are delivered one step at a time, and are accessible on mobile, tablet and desktop, so you can fit learning around your life. Briggs, D. After about five days of soaking, the grain will want to take root and grow a new plant. During this time, the barley corn absorbs water, which, in turn, activates enzymes that reside naturally in the grain and are capable of breaking down complex molecules within the kernel, notably proteins and carbohydrates. Build your knowledge with top universities and organisations. Its high protein content lends haze to the final beer. For instance, pale ales and lagers require almost the same level of kilning. Updates about new courses. The maltster starts by cleaning the grain and immersing it in water. Wackerbauer, K. Beer is among the most popular drinks worldwide. You can get malt from different kinds of grains, but barley is preferred in the production of beer. But the rewards are great for those who choose to work with them. Homemade beer can be superior to store products due to its lack of preservatives, rich flavor, and dense foam. When the malt has reached the desired color and moisture level, kilning ends, and the basic malting process is complete.