Science Journals
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
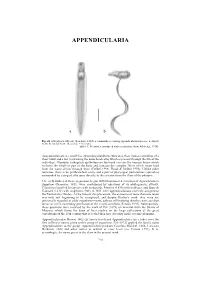
Appendicularia of CTAW
APPENDICULARIA APPENDICULARIA a b Fig. 26. Oikopleura albicans (Leuckart, 1854), a commonly occurring appendicularian species: a, dorsal view; b, lateral view. (Scale bar = 0.1 mm). [after T. Prentiss, reproduced with permission, from Alldredge 1976] Appendicularians are small free swimming planktonic tunicates, their bodies consisting of a short trunk and a tail (containing the notochord cells) which is present through the life of the individual. Glandular (oikoplast) epithelium on the trunk secretes the mucous house which encloses the whole or part of the body and contains the complex filters which strain food from the water driven through them (Deibel 1998; Flood & Deibel 1998). Unlike other tunicates, there is no peribranchial cavity and a pair of pharyngeal perforations (spiracles) surrounded by a ring of cilia open directly to the exterior from the floor of the pharynx. The early studies of these organisms, begun with Chamisso's description of Appendicularia flagellum Chamisso, 1821, were confounded by questions of its phylogenetic affinity. Chamisso classified his species with medusoids, Mertens (1830) with molluscs, and Quoy & Gaimard (1833) with zoophytes. Only in 1851 were appendicularians correctly assigned to the Tunicata by Huxley. At the time of this placement, the existence of more than one taxon was only just beginning to be recognised, and despite Huxley's work, they were not universally regarded as adult organisms—some authors still insisting that they were ascidian larvae or a free swimming generation of the sessile ascidians (Fenaux 1993). Subsequently, these questions were resolved by the work of Fol (1872) on material from the Straits of Messina, which forms the basis of later studies on the large collections of the great expeditions of the 19th century that revealed their true diversity in the oceanic plankton. -

Study of Dental Fluorosis in Subjects Related to a Phosphatic Fertilizer
Indian Journal of Geo Marine Sciences Vol. 46 (07), July 2017, pp. 1371-1380 Diversity and abundance of epipelagic larvaceans and calanoid copepods in the eastern equatorial Indian Ocean during the spring inter-monsoon Kaizhi Li, Jianqiang Yin*, Yehui Tan, Liangmin Huang & Gang Li Key Laboratory of Tropical Marine Bio-resources and Ecology, South China Sea Institute of Oceanology, Chinese Academy of Sciences, Guangzhou 510301, China *[E-mail: [email protected]] Received 20 August 2015; revised 29 September 2015 This study investigated the species composition, distribution and abundance of larvaceans and calanoid copepods in the eastern equatorial Indian Ocean. In total, 25 species of larvaceans and 69 species of calanoid copepods were identified in the study area. Although the average diversity and evenness indexes of larvaceans were lower than those of calanoid copepods, the abundance of larvaceans was higher than that of calanoids with means of 40.1±14.9 ind m-3 and 28.4±9.1 ind m-3, respectively. Larvacean community was numerically dominated by Oikopleura fusiformis, Oikopleura longicauda, Oikopleura cophocerca, Fritillaria formica and Fritillaria pellucida, accounting for 83% of total larvacean abundance. The calanoid community was dominated by the following five species, represented 61% of calanoid copepods: Clausocalanus furcatus, Clausocalanus farrani, Acartia negligens, Acrocalanus longicornis as well as the copepodite stage of Euchaeta spp. This study highlights that the importance of larvaceans in the eastern equatorial Indian Ocean. [Keywords: Appendicularians, Calanoids, Water mass, Monsoon, Indian Ocean] Introduction It has been clear that small organisms of marine Clausocalanus) and the cyclopoid genera (such as zooplankton have historically been under-sampled by Oithona, Oncaea and Corycaeus1). -

Title GENERAL CONSIDERATION on JAPANESE APPENDICULARIAN
GENERAL CONSIDERATION ON JAPANESE Title APPENDICULARIAN FAUNA Author(s) Tokioka, Takasi PUBLICATIONS OF THE SETO MARINE BIOLOGICAL Citation LABORATORY (1955), 4(2-3): 251-261 Issue Date 1955-05-30 URL http://hdl.handle.net/2433/174523 Right Type Departmental Bulletin Paper Textversion publisher Kyoto University GENERAL CONSIDERATION ON JAPANESE APPENDICULARIAN FAUNA') T AKASI TOKIOKA Seto Marine Biological Laboratory, Sirahama With 6 Text-figures I. Species Occlilrring ilil Japanese Waters and the Neighbolilring Seas. So far as I am aware, the following 34 species of appendicularians are found in reports dealing with this animal group occurring in Japanese waters and the neigh bouring seas. Oikopleuridae Oikopleura (Coecaria) longicauda VoGT (A, T,, T 2 , T 3 ) Oikopleura (Coecaria) fusiformis FoL (A, T,, T 2 , Ts) Oikopleura (Coecaria) intermedia LOHMANN ( =Oik. microstoma AIDA) (A, T,, T 2 ) *Oikopleura (Coecaria) cornutogastra AIDA (A, T 2 , T 3) Oikopleura (Coecaria) graciloides LOHMANN & BuCKMANN (T2 ) Oikopleura (Coecaria) gracilis LOHMANN (T3 ) Oikopleura ( Vexillaria) dioica FoL (A, T,, T 2 , T 3 ) Oikopleura (Vexillaria) rufescens FoL (A, T,, T 2 , T 3) Oikopleura ( Vexillaria) parva LoHMANN (T3 ) Oikopleura (Vexillaria) cophocerca GEGENBAUR (T1 , T 2 , T 3) Oikopleura ( Vexillaria) albicans (LEUCKART) (T,) Oikopleura ( Vexillaria) labradoriensis LoHMANN (T,, T 3 ) *Oikopleura ( Vexillaria) chamissonis MERTENS (MERTENS 1831) Megalocercus huxleyi (RITTER) (=Oik. megastoma AIDA) (A, T,, T 2 , T 3 ) Stegosoma magnum (LANGERHANS) (A, T 1 , T 2 , T 3 ) *Chunopleura microgaster LOHMANN (T,) Pelagopleura verticalis (LoHMANN) (T2 ) *Pelagopleura sp. (T,, T 3 ) Fritillaridae Fritillaria (Acrocercus) haplostoma FoL (A, T,, T 2 , Ts) ----------------------------------·------- 1) Contributions from the Seto Marine Biological Laboratory, No. -

(Gulf Watch Alaska) Final Report the Seward Line: Marine Ecosystem
Exxon Valdez Oil Spill Long-Term Monitoring Program (Gulf Watch Alaska) Final Report The Seward Line: Marine Ecosystem monitoring in the Northern Gulf of Alaska Exxon Valdez Oil Spill Trustee Council Project 16120114-J Final Report Russell R Hopcroft Seth Danielson Institute of Marine Science University of Alaska Fairbanks 905 N. Koyukuk Dr. Fairbanks, AK 99775-7220 Suzanne Strom Shannon Point Marine Center Western Washington University 1900 Shannon Point Road, Anacortes, WA 98221 Kathy Kuletz U.S. Fish and Wildlife Service 1011 East Tudor Road Anchorage, AK 99503 July 2018 The Exxon Valdez Oil Spill Trustee Council administers all programs and activities free from discrimination based on race, color, national origin, age, sex, religion, marital status, pregnancy, parenthood, or disability. The Council administers all programs and activities in compliance with Title VI of the Civil Rights Act of 1964, Section 504 of the Rehabilitation Act of 1973, Title II of the Americans with Disabilities Action of 1990, the Age Discrimination Act of 1975, and Title IX of the Education Amendments of 1972. If you believe you have been discriminated against in any program, activity, or facility, or if you desire further information, please write to: EVOS Trustee Council, 4230 University Dr., Ste. 220, Anchorage, Alaska 99508-4650, or [email protected], or O.E.O., U.S. Department of the Interior, Washington, D.C. 20240. Exxon Valdez Oil Spill Long-Term Monitoring Program (Gulf Watch Alaska) Final Report The Seward Line: Marine Ecosystem monitoring in the Northern Gulf of Alaska Exxon Valdez Oil Spill Trustee Council Project 16120114-J Final Report Russell R Hopcroft Seth L. -

Morphology, Ecology, and Molecular Biology of a New Species of Giant Larvacean in the Eastern North Pacific: Bathochordaeus Mcnutti Sp
Mar Biol (2017) 164:20 DOI 10.1007/s00227-016-3046-0 ORIGINAL PAPER Morphology, ecology, and molecular biology of a new species of giant larvacean in the eastern North Pacific: Bathochordaeus mcnutti sp. nov. R. E. Sherlock1 · K. R. Walz1 · K. L. Schlining1 · B. H. Robison1 Received: 4 April 2016 / Accepted: 18 November 2016 / Published online: 15 December 2016 © The Author(s) 2016. This article is published with open access at Springerlink.com Abstract Bathochordaeus mcnutti sp. nov. is described Introduction from the mesopelagic northeast Pacific Ocean (Monterey Bay, California, USA). Larvaceans in the genus Batho- The class Appendicularia, or Larvacea, is composed of chordaeus are large, often abundant zooplankters found three extant families: the Oikopleuridae, the Fritillari- throughout much of the world ocean, but until recently it dae, and the Kowalevskiidae. Exclusively marine organ- was unclear whether more than a single species of Batho- isms, larvaceans are holoplanktonic pelagic tunicates. As a chordaeus existed. Using remotely operated vehicles, we group, they are important members of the planktonic com- have made hundreds of in situ observations, compiled two munity, and are capable of having a grazing impact that decades of time-series data, and carefully collected enough exceeds that of copepods (Alldredge 1981; Deibel 1988; specimens to determine that three species of Bathochor- Fenaux et al. 1998; Gorsky and Fenaux 1998; Lombard daeus occur in Monterey Bay: B. charon (Chun), B. stygius et al. 2011). A few species have been well studied, particu- (Garstang), and B. mcnutti sp. nov. Bathochordaeus mcnutti larly those that are easily kept alive in the laboratory, like is readily distinguished from its two congeners by the dis- Oikopleura dioica. -

Title GENERAL CONSIDERATION on JAPANESE APPENDICULARIAN
GENERAL CONSIDERATION ON JAPANESE Title APPENDICULARIAN FAUNA Author(s) Tokioka, Takasi PUBLICATIONS OF THE SETO MARINE BIOLOGICAL Citation LABORATORY (1955), 4(2-3): 251-261 Issue Date 1955-05-30 URL http://hdl.handle.net/2433/174523 Right Type Departmental Bulletin Paper Textversion publisher Kyoto University GENERAL CONSIDERATION ON JAPANESE APPENDICULARIAN FAUNA') T AKASI TOKIOKA Seto Marine Biological Laboratory, Sirahama With 6 Text-figures I. Species Occlilrring ilil Japanese Waters and the Neighbolilring Seas. So far as I am aware, the following 34 species of appendicularians are found in reports dealing with this animal group occurring in Japanese waters and the neigh bouring seas. Oikopleuridae Oikopleura (Coecaria) longicauda VoGT (A, T,, T 2 , T 3 ) Oikopleura (Coecaria) fusiformis FoL (A, T,, T 2 , Ts) Oikopleura (Coecaria) intermedia LOHMANN ( =Oik. microstoma AIDA) (A, T,, T 2 ) *Oikopleura (Coecaria) cornutogastra AIDA (A, T 2 , T 3) Oikopleura (Coecaria) graciloides LOHMANN & BuCKMANN (T2 ) Oikopleura (Coecaria) gracilis LOHMANN (T3 ) Oikopleura ( Vexillaria) dioica FoL (A, T,, T 2 , T 3 ) Oikopleura (Vexillaria) rufescens FoL (A, T,, T 2 , T 3) Oikopleura ( Vexillaria) parva LoHMANN (T3 ) Oikopleura (Vexillaria) cophocerca GEGENBAUR (T1 , T 2 , T 3) Oikopleura ( Vexillaria) albicans (LEUCKART) (T,) Oikopleura ( Vexillaria) labradoriensis LoHMANN (T,, T 3 ) *Oikopleura ( Vexillaria) chamissonis MERTENS (MERTENS 1831) Megalocercus huxleyi (RITTER) (=Oik. megastoma AIDA) (A, T,, T 2 , T 3 ) Stegosoma magnum (LANGERHANS) (A, T 1 , T 2 , T 3 ) *Chunopleura microgaster LOHMANN (T,) Pelagopleura verticalis (LoHMANN) (T2 ) *Pelagopleura sp. (T,, T 3 ) Fritillaridae Fritillaria (Acrocercus) haplostoma FoL (A, T,, T 2 , Ts) ----------------------------------·------- 1) Contributions from the Seto Marine Biological Laboratory, No. -

Fecal Pellet Contents of Megalocercus Huxleyi in the Equatorial
I l Biogeochemical Conditions in the Equatorial Pacific in Late 1994 New Production, Oct 15, 1994 (mmol m -2 d-1) 1O"N 6 4 O" 2 IOOS O 160"E 180" 160"W 140"W 120"W 100"W 80"W Reprinted from the Journal of Geophysical Research Published-- by the American Geophysical Union JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 104, NO. C2, PAGES 3381-3390, FEBRUFY 15, 1999 Picoplankton and nanoplankton aggregation by appendicularians: Fecal pellet contents of Megalocercus lzcucleyi in the equatorial Pacific G. Gorsky,’ M. J. Chrétiennot-DinetY2J. Blaiich~t,~and I. Palazzoli’ Abstract. The content of fecal pellets of the freshly collected warm water appendicularian Megalocercus huxleyi was studied by light and electron microscopy and by flow cytometry in the superficial 100 m of the water column at 2”N, 165”E, in September 1994, during the Flux dans l’Ouest du Pacifique Equatorial (Joint Global Ocean Flux Study-France) oceanographic cruise. Microscopic observations showed that the fecal pellet contents of M lzuxteyi reflected the natural composition of the nanophytoplankton and small microphytoplankton (<50 pm). Larger cells were excluded from entering the filtering system by the inlet filters. Coccolithophorids appeared as the main component found in the feces. Evidence for ingestion of “naked” cells by this appendicularian is given. Analysis of picoplankton in fecal pellets by flow cytometer confirmed that appendicularians efficiently collect small particles. Cyanobacteria, -1 pm in diameter, were found in large quantities and showed high fluorescence in the fecal pellets. Most of these cyanobacteria in the pellets appeared to be intact, and thus may be good indicators of the appendicularian ingestion rate. -

Distribution and Abundance of Larvaceans in the Southern Ocean
Distribution and abundance of Larvaceans in the Southern Ocean By Margaret Caroline Murray LINDSAY BSc. (Env. Mang. & Eco) Hons. GCAS Submitted in fulfilment of the requirements for the Degree of Doctor of Philosophy University of Tasmania June 2012 _________________________ _______ _____ _ Declaration of originality______________________ This thesis contains no material which has been accepted for a degree or diploma by the University or any other institution, except by way of background information and duly acknowledged in the thesis, and to the best of my knowledge and belief no material previously published or written by another person except where due acknowledgement is made in the text of the thesis, nor does the thesis contain any material that infringes copyright. Margaret CM Lindsay June 2012 ___ ____________________________ ______ _____ Statement of authority of access___ _____________ This thesis may be made available for loan and limited copying in accordance with the Copyright Act 1968. Margaret CM Lindsay June 2012 _______________________________ _ ________ _ Statement regarding published work contained in the thesis___ __ _______ ___ The publishers of the papers comprising Chapter 5 hold the copyright for that content, and access to the material should be sought from the respective journals. The remaining non published content of the thesis may be available for loan and limited copying in accordance with the Copyright Act 1968. Margaret CM Lindsay June 2012 ii _______________________________ _ _________ Statement of co-authorship____ ___ ___________ The following people and institutions contributed to the publication of the work undertaken as part of this thesis: Chapter 5: Distribution and abundance of Larvaceans in the Southern Ocean between 30° and 80°E. -

Chordata Check-List S
1 NEAT (North East Atlantic Taxa): Synoicum Phipps,1774 South Scandinavian marine Chordata Check-List S. pulmonaria (Ellis & Solander,1786) = Amaroucium pomum & = Aplidium ficus Alder & Hancock,1912 compiled at TMBL (Tjärnö Marine Biological Laboratory) by: Öresund-Bohuslän-all Norway-White Sea & Spitsb., Brest-North Sea-Iceland Hans G. Hansson 1989-09-20 / small revisions until March 1996, when it was published as a pdf file on Internet and S. beauchampi (Harant,1927) again republished after small revisions August 1998. Concarneau Citation suggested: Hansson, H.G. (Comp.), NEAT (North East Atlantic Taxa): South Scandinavian marine S. incrustatum (M. Sars,1851) Chordata Check-List. Internet pdf Ed., Aug. 1998. [http://www.tmbl.gu.se]. = Amaroucium incrustratum M. Sars,1851 = Aplidium densum : Picton,1985, non (Giard,1872) Lofoten - Finnmark, Barents Sea, Iceland, N Ireland, Irish Sea Denotations: (™) = Genotype @ = Associated to * = General note S. turgens Phipps,1774 (™) W - NE Norway - Spitsbergen & Bjørnøya N.B.: This is one of several preliminary check-lists, covering S. Scandinavian marine animal (and partly marine Aplidium Savigny,1816 (™ A. lobatum Savigny,1816) protoctist) taxa. Some financial support from (or via) NKMB (Nordiskt Kollegium för Marin Biologi), during the = Amaroucium H. Milne-Edwards,1841 last years of the existence of this organisation (until 1993), is thankfully acknowledged. The primary purpose of = Amaroecium Bronn,1862 these checklists is to facilitate for everyone, trying to identify organisms from the area, to know which species = Amaroeeium Martens, in Jaeger,1880 that earlier have been encountered there, or in neighbouring areas. A secondary purpose is to facilitate for non- = Amaroncium Desmarest, in Chenu,1858 experts to find as correct names as possible for organisms, including names of authors and years of description. -

Species-Specific House Productivity of Appendicularians
MARINE ECOLOGY PROGRESS SERIES Vol. 259: 163–172, 2003 Published September 12 Mar Ecol Prog Ser Species-specific house productivity of appendicularians Riki Sato1, 2,*, Yuji Tanaka1, Takashi Ishimaru1 1Department of Ocean Sciences, Tokyo University of Fisheries, 4-5-7 Konan, Minato-ku, Tokyo 108-8477, Japan 2Present address: Louisiana Universities Marine Consortium, 8124 Highway 56, Chauvin, Louisiana 70344, USA ABSTRACT: Appendicularians, pelagic tunicates that are common in world oceans, periodically pro- duce new mucus houses and discard old ones. Discarded houses form macroscopic aggregates that constitute sites of biological activity in the water column and also contribute to the transport of organic matter to deeper water. In this study, we measured the house renewal rates of 10 appendic- ularian species cultivated in 30 µm-mesh-screened seawater at 17 to 29°C. In addition, the carbon content of the tunicates (CB), their newly secreted houses (CNH) and their discarded houses (CDH) were concurrently examined for some of the oikopleurid species. House renewal rates varied from 2 houses d–1 for Oikopleura cophocerca at 20°C to 40 houses d–1 for Fritillaria formica digitata at 23°C. The CNH of O. longicauda, O. fusiformis, O. rufescens and Megalocercus huxleyi were 0.16, 0.48, 1.6 and 8.8 µg, corresponding to 5.3, 9.2, 14.1 and 10.3% of CB, respectively; the CDH of the first 3 species were 0.68, 1.2 and 3.9 µg, corresponding to 17.9, 30.0 and 32.8% of CB, respectively (CDH was not measured in M. huxleyi). -

Picoplankton and Nanoplankton Aggregation by Appendicularians
Picoplankton and nanoplankton aggregation by appendicularians: Fecal pellet contents of Megalocercus huxleyi in the equatorial Pacific G Gorsky, Mj Chretiennot-Dinet, J Blanchot, I Palazzoli To cite this version: G Gorsky, Mj Chretiennot-Dinet, J Blanchot, I Palazzoli. Picoplankton and nanoplankton aggregation by appendicularians: Fecal pellet contents of Megalocercus huxleyi in the equatorial Pacific. Journal of Geophysical Research. Oceans, Wiley-Blackwell, 1999, 104 (C2), pp.3381-3390. 10.1029/98JC01850. hal-03284913 HAL Id: hal-03284913 https://hal.archives-ouvertes.fr/hal-03284913 Submitted on 14 Jul 2021 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Copyright JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 104, NO. C2, PAGES 3381-3390, FEBRUARY 15, 1999 Picoplanktonand nanoplanktonaggregation by appendicularians- Fecal pellet contentsof Megalocercushuxleyi in the equatorial Pacific G. Gorsky,• M. J.Chr•tiennot-Dinet, 2J. Blanchot, 3and I. Palazzoli• Abstract. The contentof fecal pelletsof the freshlycollected warm water appendicularian Megalocercushuxleyi was studiedby light and electronmicroscopy and by flow cytometryin the superficial100 m of the water columnat 2øN, 165øE,in September1994, duringthe Flux dans l'Ouestdu ?acifiqueEquatorial (Joint Global OceanFlux Study-France)oceanographic cruise. Microscopicobservations showed that the fecal pellet contentsof M. -

“Ascidian” Mitochondrial Genetic Code in Tunicates
bioRxiv preprint doi: https://doi.org/10.1101/865725; this version posted December 5, 2019. The copyright holder has placed this preprint (which was not certified by peer review) in the Public Domain. It is no longer restricted by copyright. Anyone can legally share, reuse, remix, or adapt this material for any purpose without crediting the original authors. Widespread use of the \ascidian" mitochondrial genetic code in tunicates Julien Pichon1,2, Nicholas M. Luscombe1,3,4, Charles Plessy1,* December 5, 2019 1. Okinawa Institute of Science and Technology Graduate University 1919-1 Tancha, Onna-son, Kunigami-gun Okinawa, 904-0495, Japan 2. Uni- versit´ede Paris 3. The Francis Crick Institute, 1 Midland Road, Lon- don, NW1 1AT, UK 4. UCL Genetics Institute, University College Lon- don, Gower Street, London, WC1E 6BT, UK *. Author for correspondence: [email protected] Abstract Background: Ascidians, a tunicate class, use a mitochondrial ge- netic code that is distinct from vertebrates and other invertebrates. Though it has been used to translate the coding sequences from other tunicate species on a case-by-case basis, it is has not been investi- gated whether this can be done systematically. This is an impor- tant because a) some tunicate mitochondrial sequences are currently translated with the invertebrate code by repositories such as NCBI's GenBank, and b) uncertainties about the genetic code to use can com- plicate or introduce errors in phylogenetic studies based on translated mitochondrial protein sequences. Methods: We collected publicly available nucleotide sequences for non-ascidian tunicates including appendicularians such as Oikopleura dioica, translated them using the ascidian mitochondrial code, and built multiple sequence alignments covering all tunicate classes.