Functional Morphology of Terrestrial Prey Capture in Salamandrid Salamanders Charlotte M
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Title a New Species of Pachytriton from China (Amphibia: Urodela
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Kyoto University Research Information Repository A New Species of Pachytriton from China (Amphibia: Urodela: Title Salamandridae) Author(s) Nishikawa, Kanto; Matsui, Masafumi; Jiang, Jian-Ping Citation Current Herpetology (2012), 31(1): 21-27 Issue Date 2012-06 URL http://hdl.handle.net/2433/216840 Right © 2012 by The Herpetological Society of Japan Type Journal Article Textversion publisher Kyoto University Current Herpetology 31(1): 21–27, June 2012 doi 10.5358/hsj.31.21 © 2012 by The Herpetological Society of Japan A New Species of Pachytriton from China (Amphibia: Urodela: Salamandridae) 1 1 2 KANTO NISHIKAWA *, MASAFUMI MATSUI , AND JIAN-PING JIANG 1 Graduate School of Human and Environmental Studies, Kyoto University, Yoshida Nihonmatsu-cho, Sakyo-ku, Kyoto 606–8501, Japan 2 Chengdu Institute of Biology, Chinese Academy of Sciences, Chengdu 610041, China Abstract: A new species of the salamandrid genus Pachytriton is described based on two individuals purchased from pet shops in Japan. The original locality of these specimens is known only as “China”, and further details are not known. Morphologically, this species differs from all other congeners in the combination of coloration, body size, snout length, head width, tail length and width, and length of upper jaw tooth series and vomerine tooth series. Genetically, this species is separated from all other congeners by substantial genetic distances in mitochondrial DNA sequences. Key words: China; DNA bar-coding; New species; Pachytriton; Pet trade INTRODUCTION After these specimens were obtained from two pet shops in Japan in 2007, we tried to The salamandrid genus Pachytriton Bou- discover their origin by visiting China every lenger, 1878, occurs in eastern and southeast- year to collect specimens in the wild, but with- ern China (Fei et al., 2006), and currently out success. -

Notophthalmus Perstriatus) Version 1.0
Species Status Assessment for the Striped Newt (Notophthalmus perstriatus) Version 1.0 Striped newt eft. Photo credit Ryan Means (used with permission). May 2018 U.S. Fish and Wildlife Service Region 4 Jacksonville, Florida 1 Acknowledgements This document was prepared by the U.S. Fish and Wildlife Service’s North Florida Field Office with assistance from the Georgia Field Office, and the striped newt Species Status Assessment Team (Sabrina West (USFWS-Region 8), Kaye London (USFWS-Region 4) Christopher Coppola (USFWS-Region 4), and Lourdes Mena (USFWS-Region 4)). Additionally, valuable peer reviews of a draft of this document were provided by Lora Smith (Jones Ecological Research Center) , Dirk Stevenson (Altamaha Consulting), Dr. Eric Hoffman (University of Central Florida), Dr. Susan Walls (USGS), and other partners, including members of the Striped Newt Working Group. We appreciate their comments, which resulted in a more robust status assessment and final report. EXECUTIVE SUMMARY This Species Status Assessment (SSA) is an in-depth review of the striped newt's (Notophthalmus perstriatus) biology and threats, an evaluation of its biological status, and an assessment of the resources and conditions needed to maintain species viability. We begin the SSA with an understanding of the species’ unique life history, and from that we evaluate the biological requirements of individuals, populations, and species using the principles of population resiliency, species redundancy, and species representation. All three concepts (or analogous ones) apply at both the population and species levels, and are explained that way below for simplicity and clarity as we introduce them. The striped newt is a small salamander that uses ephemeral wetlands and the upland habitat (scrub, mesic flatwoods, and sandhills) that surrounds those wetlands. -

Zootaxa, a New Species of Paramesotriton (Caudata
Zootaxa 1775: 51–60 (2008) ISSN 1175-5326 (print edition) www.mapress.com/zootaxa/ ZOOTAXA Copyright © 2008 · Magnolia Press ISSN 1175-5334 (online edition) A new species of Paramesotriton (Caudata: Salamandridae) from Guizhou Province, China HAITAO ZHAO1, 2, 5, JING CHE2,5, WEIWEI ZHOU2, YONGXIANG CHEN1, HAIPENG ZHAO3 & YA-PING ZHANG2 ,4 1Department of Environment and Life Science, Bijie College, Guizhou 551700, China 2State Key Laboratory of Genetic Resources and Evolution, Kunming Institute of Zoology, the Chinese Academy of Sciences, Kunming 650223, China 3School of Life Science, Southwest University, Chongqing 400715, China 4Corresponding authors. E-mail: [email protected] 5 These authors contributed equally to this work. Abstract We describe a new species of salamander, Paramesotriton zhijinensis, from Guizhou Province, China. The generic allo- cation of the new species is based on morphological and molecular characters. In morphology, it is most similar to Paramesotriton chinensis but differs in having distinct gland emitting a malodorous secretion (here named scent gland), a postocular stripe, and two non-continuous, dorsolateral stripes on the dorsolateral ridges. Furthermore, neoteny was observed in most individuals of the new species. This has not been previously reported to occur in any other species of Paramesotriton. Analysis of our molecular data suggests that this species a third major evolutionary lineage in the genus Paramesotriton. Key words: Caudata; Salamandridae; Paramesotriton zhijinensis; new species; scent gland; Guizhou; China Introduction Guizhou Province, located in the southwestern mountainous region of China, is known for its rich amphibian faunal diversity (Liu and Hu 1961). During recent surveys of the Guizhou herpetofauna (July, September, and November, 2006; January and September, 2007), we collected salamanders superficially resembling Parame- sotriton chinensis (Gray). -

Summary Report of Freshwater Nonindigenous Aquatic Species in U.S
Summary Report of Freshwater Nonindigenous Aquatic Species in U.S. Fish and Wildlife Service Region 4—An Update April 2013 Prepared by: Pam L. Fuller, Amy J. Benson, and Matthew J. Cannister U.S. Geological Survey Southeast Ecological Science Center Gainesville, Florida Prepared for: U.S. Fish and Wildlife Service Southeast Region Atlanta, Georgia Cover Photos: Silver Carp, Hypophthalmichthys molitrix – Auburn University Giant Applesnail, Pomacea maculata – David Knott Straightedge Crayfish, Procambarus hayi – U.S. Forest Service i Table of Contents Table of Contents ...................................................................................................................................... ii List of Figures ............................................................................................................................................ v List of Tables ............................................................................................................................................ vi INTRODUCTION ............................................................................................................................................. 1 Overview of Region 4 Introductions Since 2000 ....................................................................................... 1 Format of Species Accounts ...................................................................................................................... 2 Explanation of Maps ................................................................................................................................ -

Variations in Tetrodotoxin Levels in Populations of Taricha Granulosa
www.nature.com/scientificreports OPEN Variations in tetrodotoxin levels in populations of Taricha granulosa are expressed in the morphology of their cutaneous glands Pedro Luiz Mailho-Fontana1*, Carlos Jared1, Marta Maria Antoniazzi1, Juliana Mozer Sciani 2, Daniel Carvalho Pimenta 1, Amber N. Stokes3, Taran Grant4, Edmund D. Brodie III5 & Edmund D. Brodie Jr.6 Tetrodotoxin (TTX), one of the most toxic substances in nature, is present in bacteria, invertebrates, fshes, and amphibians. Marine organisms seem to bioaccumulate TTX from their food or acquire it from symbiotic bacteria, but its origin in amphibians is unclear. Taricha granulosa can exhibit high TTX levels, presumably concentrated in skin poison glands, acting as an agent of selection upon predatory garter snakes (Thamnophis). This co-evolutionary arms race induces variation in T. granulosa TTX levels, from very high to undetectable. Using morphology and biochemistry, we investigated diferences in toxin localization and quality between two populations at the extremes of toxicity. TTX concentration within poison glands is related to the volume of a single cell type in which TTX occurs exclusively in distinctive secretory granules, suggesting a relationship between granule structure and chemical composition. TTX was detected in mucous glands in both populations, contradicting the general understanding that these glands do not secrete defensive chemicals and expanding currently held interpretations of amphibian skin gland functionality. Skin secretions of the two populations difered in low-mass molecules and proteins. Our results demonstrate that interpopulation variation in TTX levels is related to poison gland morphology. Tetrodotoxin (TTX) is one of the most toxic and well-studied but still mysterious natural products. -

Maximum Size of the Spectacled Salamander, Salamandrina
©Österreichische Gesellschaft für Herpetologie e.V., Wien, Austria, download unter www.biologiezentrum.at 186 SHORT NOTE HERPETOZOA 18 (3/4) Wien, 30. Dezember 2005 SHORT NOTE western Honduras.- Senckenbergiana biologica, ratios than females (e.g., head length/ snout- Frankfurt am Main; 81(1/2): 269-276. MARTINS, M. & vent length, tail length/snout-vent length) as OLIVEIRA, E. (1999): Natural history of snakes in forests of the Manaus Region, Central Amazonia, Brazil.- reported by VANNI (1980), ZUFFI (1999) and Herpetological Natural History, Phoenix; 6 (2):78-150. ANTONELLI (1999). Total length (TL; i.e. PEREZ-SANTOS, C. & MORENO, A. (1990): Anotaciones from the tip of the snout to the end of the tail) biométricas y alimenticias de la serpiente neotropical of the Spectacled Salamander is usually 8-10 Atractus badius (BOIE, 1827) (Serpentes, Colubridae) de la Colección del Museo Nacional de Ciencias Naturales cm (VANNI 1980; ZUFFI 1999; ANGELINI et al. de Madrid.- Revista Espanola de Herpetologia, Madrid; 2001). Until 2001 the maximum TL record- 4: 9-15. PETERS, J. A. (1960): The snakes of the sub- ed in males and females were 9.6 cm family Dipsadinae.- Miscellaneous Publications, (ANTONELLI 1999) and 11.6 cm, respectively Museum of Zoology, Univ. of Michigan, Ann Arbor; (114): 1-224. SAVAGE, J. (1960): A revision of the (ZAGAGLIONI 1978; VANNI 1980). Later, Ecuadorian snakes of the colubrid genus Atractus.- ANGELINI et al. (2001) reported a maximum Miscellaneous Publications, Museum of Zoology, Univ TL of 12.3 cm recorded in two females from of Michigan, Ann Arbor; (112): 1-86. different populations of S. -

<I>Salamandra Salamandra</I>
International Journal of Speleology 46 (3) 321-329 Tampa, FL (USA) September 2017 Available online at scholarcommons.usf.edu/ijs International Journal of Speleology Off icial Journal of Union Internationale de Spéléologie Subterranean systems provide a suitable overwintering habitat for Salamandra salamandra Monika Balogová1*, Dušan Jelić2, Michaela Kyselová1, and Marcel Uhrin1,3 1Institute of Biology and Ecology, Faculty of Science, P. J. Šafárik University, Šrobárova 2, 041 54 Košice, Slovakia 2Croatian Institute for Biodiversity, Lipovac I., br. 7, 10000 Zagreb, Croatia 3Department of Forest Protection and Wildlife Management, Faculty of Forestry and Wood Sciences, Czech University of Life Sciences, Kamýcká 1176, 165 21 Praha, Czech Republic Abstract: The fire salamander (Salamandra salamandra) has been repeatedly noted to occur in natural and artificial subterranean systems. Despite the obvious connection of this species with underground shelters, their level of dependence and importance to the species is still not fully understood. In this study, we carried out long-term monitoring based on the capture-mark- recapture method in two wintering populations aggregated in extensive underground habitats. Using the POPAN model we found the population size in a natural shelter to be more than twice that of an artificial underground shelter. Survival and recapture probabilities calculated using the Cormack-Jolly-Seber model were very constant over time, with higher survival values in males than in females and juveniles, though in terms of recapture probability, the opposite situation was recorded. In addition, survival probability obtained from Cormack-Jolly-Seber model was higher than survival from POPAN model. The observed bigger population size and the lower recapture rate in the natural cave was probably a reflection of habitat complexity. -

<I>In Vivo</I> Sexual Discrimination in <I>Salamandrina Perspicillata</I
HERPETOLOGICAL JOURNAL 20: 17–24, 2010 In vivo sexual discrimination in Salamandrina perspicillata: a cross-check analysis of annual changes in external cloacal morphology and spermic urine release Leonardo Vignoli1, Romina Silici1, Rossana Brizzi2 & Marco A. Bologna1 1Dipartimento di Biologia Ambientale, Università Roma Tre, Rome, Italy 2Dipartimento di Biologia Evoluzionistica “Leo Pardi”, Università di Firenze, Florence, Italy In Salamandrina, the lack of visible external sexual dimorphism makes the sexing of individuals difficult without sacrifice. The cloaca of Salamandrina in both males and females appears externally as a slit on an unswollen surface, a trait which is consistent throughout the year. Nonetheless, a slight divarication of its borders allows the recognition of three morphs (A, B and C), respectively characterizing male cloaca (all phases), female cloaca without protruding oviductal papillae (courtship phase) and female cloaca with prolapsed oviductal papillae (oviposition phase). Figures and schematic diagrams are provided to illustrate the differences in detail, which are all recognizable to the naked eye or by means of a hand magnifier. In addition to morphology, another reliable method of sexing salamanders is urine examination, albeit only during the courtship and post-courtship phases. Applying these methods for sex determination, we found a male- biased operational sex ratio in two populations, ranging from 6.6:1 (autumn–winter) to 14:1 (May). Males were confined to terrestrial environments, whereas females were also found in water during oviposition. Salamandrina perspicillata was active throughout the year, except during the hottest months (July–August). Key words: cloaca, salamander, sex determination, sex ratio, sexual dimorphism INTRODUCTION Hayes, 1998). Sexing based on behavioural differences requires direct observations, which often prove difficult. -

Is the Italian Stream Frog (Rana Italica Dubois, 1987) an Opportunistic Exploiter of Cave Twilight Zone?
A peer-reviewed open-access journal Subterranean IsBiology the Italian 25: 49–60 stream (2018) frog (Rana italica Dubois, 1987) an opportunistic exploiter... 49 doi: 10.3897/subtbiol.25.23803 SHORT COMMUNICATION Subterranean Published by http://subtbiol.pensoft.net The International Society Biology for Subterranean Biology Is the Italian stream frog (Rana italica Dubois, 1987) an opportunistic exploiter of cave twilight zone? Enrico Lunghi1,2,3, Giacomo Bruni4, Gentile Francesco Ficetola5,6, Raoul Manenti5 1 Universität Trier Fachbereich VI Raum-und Umweltwissenschaften Biogeographie, Universitätsring 15, 54286 Trier, Germany 2 Museo di Storia Naturale dell’Università di Firenze, Sezione di Zoologia “La Spe- cola”, Via Romana 17, 50125 Firenze, Italy 3 Natural Oasis, Via di Galceti 141, 59100 Prato, Italy 4 Vrije Universiteit Brussel, Boulevard de la Plaine 2, 1050 Ixelles, Bruxelles, Belgium 5 Department of Environmen- tal Science and Policy, Università degli Studi di Milano, Via Celoria 26, 20133 Milano, Italy 6 Univ. Greno- ble Alpes, CNRS, Laboratoire d’Écologie Alpine (LECA), F-38000 Grenoble, France Corresponding author: Enrico Lunghi ([email protected]) Academic editor: O. Moldovan | Received 22 January 2018 | Accepted 9 March 2018 | Published 20 March 2018 http://zoobank.org/07A81673-E845-4056-B31C-6EADC6CA737C Citation: Lunghi E, Bruni G, Ficetola GF, Manenti R (2018) Is the Italian stream frog (Rana italica Dubois, 1987) an opportunistic exploiter of cave twilight zone? Subterranean Biology 25: 49–60. https://doi.org/10.3897/subtbiol.25.23803 Abstract Studies on frogs exploiting subterranean environments are extremely scarce, as these Amphibians are usually considered accidental in these environments. However, according to recent studies, some anurans actively select subterranean environments on the basis of specific environmental features, and thus are able to inhabit these environments throughout the year. -

The Evolution of Pueriparity Maintains Multiple Paternity in a Polymorphic Viviparous Salamander Lucía Alarcón‑Ríos 1*, Alfredo G
www.nature.com/scientificreports OPEN The evolution of pueriparity maintains multiple paternity in a polymorphic viviparous salamander Lucía Alarcón‑Ríos 1*, Alfredo G. Nicieza 1,2, André Lourenço 3,4 & Guillermo Velo‑Antón 3* The reduction in fecundity associated with the evolution of viviparity may have far‑reaching implications for the ecology, demography, and evolution of populations. The evolution of a polygamous behaviour (e.g. polyandry) may counteract some of the efects underlying a lower fecundity, such as the reduction in genetic diversity. Comparing patterns of multiple paternity between reproductive modes allows us to understand how viviparity accounts for the trade-of between ofspring quality and quantity. We analysed genetic patterns of paternity and ofspring genetic diversity across 42 families from two modes of viviparity in a reproductive polymorphic species, Salamandra salamandra. This species shows an ancestral (larviparity: large clutches of free aquatic larvae), and a derived reproductive mode (pueriparity: smaller clutches of larger terrestrial juveniles). Our results confrm the existence of multiple paternity in pueriparous salamanders. Furthermore, we show the evolution of pueriparity maintains, and even increases, the occurrence of multiple paternity and the number of sires compared to larviparity, though we did not fnd a clear efect on genetic diversity. High incidence of multiple paternity in pueriparous populations might arise as a mechanism to avoid fertilization failures and to ensure reproductive success, and thus has important implications in highly isolated populations with small broods. Te evolution of viviparity entails pronounced changes in individuals’ reproductive biology and behaviour and, by extension, on population dynamics1–3. For example, viviparous species ofen show an increased parental invest- ment compared to oviparous ones because they produce larger and more developed ofspring that are protected from external pressures for longer periods within the mother 4–7. -

D. Bruce Means
D. Bruce Means Scientific and Technical Publications, Popular Articles, and Contract Reports 1. Means, D. Bruce and Clive J. Longden. 1970. Observations on the occurrence of Desmognathus monticola in Florida. Herpetologica 26(4):396-399. 2. Means, D. Bruce. 1971. Dentitional morphology in desmognathine salamanders (Amphibia: Plethodontidae). Association of Southeastern Biologists Bulletin 18(2):45. (Abstr.) 3. Means, D. Bruce. 1972a. Notes on the autumn breeding biology of Ambystoma cingulatum (Cope) (Amphibia: Urodela: Ambystomatidae). Association of Southeastern Biologists Bulletin 19(2):84. (Abstr.) 4. Means, D. Bruce. 1972b. Osteology of the skull and atlas of Amphiuma pholeter Neill (Amphibia: Urodela: Amphiumidae). Association of Southeastern Biologists Bulletin 19(2):84. (Abstr.) 5. Hobbs, Horton H., Jr. and D. Bruce Means. 1972c. Two new troglobitic crayfishes (Decapoda, Astacidae) from Florida. Proceedings of the Biological Society of Washington 84(46):393-410. 6. Means, D. Bruce. 1972d. Comments on undivided teeth in urodeles. Copeia 1972(3):386-388. 7. Means, D. Bruce. 1974a. The status of Desmognathus brimleyorum Stejneger and an analysis of the genus Desmognathus in Florida. Bulletin of the Florida State Museum, Biological Sciences, 18(1):1-100. 8. Means, D. Bruce. 1974b. City of Tallahassee Powerline Project: Faunal Impact Study. Report under contract with the City of Tallahassee, Florida, 198 pages. (Contract report.) 9. Means, D. Bruce. 1974c. A survey of the amphibians, reptiles and mammals inhabiting St. George Island, Franklin County, Florida with comments on vulnerable aspects of their ecology. 21 pages in R. J. Livingston and N. M. Thompson, editors. Field and laboratory studies concerning effects of various pollutants on estuarine and coastal organisms with application to the management of the Apalachicola Bay system (North Florida, U.S.A.). -
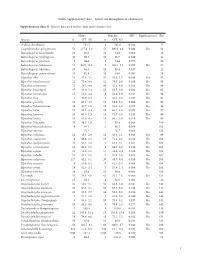
I Online Supplementary Data – Sexual Size Dimorphism in Salamanders
Online Supplementary data – Sexual size dimorphism in salamanders Supplementary data S1. Species data used in this study and references list. Males Females SSD Significant test Ref Species n SVL±SD n SVL±SD Andrias davidianus 2 532.5 8 383.0 -0.280 12 Cryptobranchus alleganiensis 53 277.4±5.2 52 300.9±3.4 0.084 Yes 61 Batrachuperus karlschmidti 10 80.0 10 84.8 0.060 26 Batrachuperus londongensis 20 98.6 10 96.7 -0.019 12 Batrachuperus pinchonii 5 69.6 5 74.6 0.070 26 Batrachuperus taibaiensis 11 92.9±12.1 9 102.1±7.1 0.099 Yes 27 Batrachuperus tibetanus 10 94.5 10 92.8 -0.017 12 Batrachuperus yenyuadensis 10 82.8 10 74.8 -0.096 26 Hynobius abei 24 57.8±2.1 34 55.0±1.2 -0.048 Yes 92 Hynobius amakusaensis 22 75.4±4.8 12 76.5±3.6 0.014 No 93 Hynobius arisanensis 72 54.3±4.8 40 55.2±4.8 0.016 No 94 Hynobius boulengeri 37 83.0±5.4 15 91.5±3.8 0.102 Yes 95 Hynobius formosanus 15 53.0±4.4 8 52.4±3.9 -0.011 No 94 Hynobius fuca 4 50.9±2.8 3 52.8±2.0 0.037 No 94 Hynobius glacialis 12 63.1±4.7 11 58.9±5.2 -0.066 No 94 Hynobius hidamontanus 39 47.7±1.0 15 51.3±1.2 0.075 Yes 96 Hynobius katoi 12 58.4±3.3 10 62.7±1.6 0.073 Yes 97 Hynobius kimurae 20 63.0±1.5 15 72.7±2.0 0.153 Yes 98 Hynobius leechii 70 61.6±4.5 18 66.5±5.9 0.079 Yes 99 Hynobius lichenatus 37 58.5±1.9 2 53.8 -0.080 100 Hynobius maoershanensis 4 86.1 2 80.1 -0.069 101 Hynobius naevius 72.1 76.7 0.063 102 Hynobius nebulosus 14 48.3±2.9 12 50.4±2.1 0.043 Yes 96 Hynobius osumiensis 9 68.4±3.1 15 70.2±3.0 0.026 No 103 Hynobius quelpaertensis 41 52.5±3.8 4 61.3±4.1 0.167 Yes 104 Hynobius