Antisense Suppression of a (+)-D-Cadinene Synthase Gene In
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Normas Para Confecção Da Versão
UNIVERSIDADE FEDERAL DE UBERLÂNDIA INSTITUTO DE GENÉTICA E BIOQUÍMICA PÓS-GRADUAÇÃO EM GENÉTICA E BIOQUÍMICA Poliploidia e variações reprodutivas em Bombacoideae (Malvaceae): distribuição geográfica, filogeografia e tamanho do genoma Aluna: Rafaela Cabral Marinho Orientadora: Profª. Drª. Ana Maria Bonetti Co-orientador: Prof. Dr. Paulo Eugênio Alves Macedo de Oliveira UBERLÂNDIA - MG 2017 UNIVERSIDADE FEDERAL DE UBERLÂNDIA INSTITUTO DE GENÉTICA E BIOQUÍMICA PÓS-GRADUAÇÃO EM GENÉTICA E BIOQUÍMICA Poliploidia e variações reprodutivas em Bombacoideae (Malvaceae): distribuição geográfica, filogeografia e tamanho do genoma Aluna: Rafaela Cabral Marinho Orientadora: Profª. Drª. Ana Maria Bonetti Co-orientador: Prof. Dr. Paulo Eugênio Alves Macedo de Oliveira Tese apresentada à Universidade Federal de Uberlândia como parte dos requisitos para obtenção do Título de Doutora em Genética e Bioquímica (Área Genética) UBERLÂNDIA – MG 2017 ii Dados Internacionais de Catalogação na Publicação (CIP) Sistema de Bibliotecas da UFU, MG, Brasil. M338p Marinho, Rafaela Cabral, 1988 2017 Poliploidia e variações reprodutivas em Bombacoideae (Malvaceae): distribuição geográfica, filogeografia e tamanho do genoma / Rafaela Cabral Marinho. - 2017. 100 f. : il. Orientadora: Ana Maria Bonetti. Coorientador: Paulo Eugênio Alves Macedo de Oliveira. Tese (doutorado) - Universidade Federal de Uberlândia, Programa de Pós-Graduação em Genética e Bioquímica. Disponível em: http://dx.doi.org/10.14393/ufu.di.2018.134 Inclui bibliografia. 1. Genética - Teses. 2. Malvaceae -

Yarra Yarra Group Inc (Incorporation No
Australian Plants Society Yarra Yarra Group Inc (Incorporation No. A0039676Y) Newsletter May 2018 May 3: Maree & Graham Goods : Gardening in the Wimmera Graham & Maree come from a farming and administration background. They are Life Members of the Wimmera Growers of Australian Plants of which they have been members for over 40 years. Their garden has been open to the public on several occasions for various charities. They ran a wholesale nursery for several years specialising in Eremophilas. Graham and Maree’s current project is growing plants, landscaping and overseeing the planting of the gardens surrounding the new building of their Church which was opened recently. They first became involved with Australia’s inland deserts in 2002 with an organisation called Desert Discovery as part of the Botany team. In 2008, Maree became the Botany Team Leader, while Graham was involved with the photography and identification of specimens. They have done volunteer work for Australian Wildlife Conservancy, Friends of the Great Victoria Desert, Victorian and Western Australian Herbariums in collecting, identifying and processing specimens. Graham and Maree are co-authors of the book, Birds and Plants of the Little Desert, a photographic guide and Maree a co-author of Australia’s Eremophilas Website: apsyarrayarra.org.au Facebook: facebook.com/APSYarraYarra Email: [email protected] | 1 APS Yarra Yarra Particulars APS YY General Meeting APS YY Garden Visits: Speakers: May 13, 2 pm, Bill Aitchison and Sue Guymer Garden, Donvale. 7-June Greg Moore Urban Greening 5-July Ryan Phillips Animal Interactions Natural bush garden with ponds,stream and large dam merging to adjacent bush. -

Growing Australian Plants
ASSOqIATTON OF SOCIETIES FOR GROWING AUSTRALIAN PLANTS LETTER NO 11 :ISSN: 1488-1488 at Hibiscus species H. forsterii F.D. Wilson (Sect. Furcaria DC') Contaitrer srown guclerim (jueensland 6n May 2007 Images C' Harve) G. Harvey Hibiscus diversifolius winter bloom 2nd June 2007. lnage NL 1l p.l Welcome to Newsletter No I l The front page depicts H. forsterii from the Cape York region of Queensland. The image of H. diversifolius blooms at the foot of the page contrast sharply with the purple flowers obtained in summer ; (see front page of Newsletter No 10). When the wirrter temperature is not quite so cold the off-white colour seen in the image, tends towards pale lemon and with still warmer weather becomes pink and finally red/purple in summer. It is being sold in Fairhill Native Plant Nursery as "Colour Magic". The so called'Norfolk Island Hibiscus" : Lagunaria patersonius (Andrews) G. Don subsp. patersonius, has appeared on a Norfolk Island postage stamp as illustrated bottom right on the front page. Winter arrived with a vengeance in the first week of June following a very mild autumn. Many Hibiscus came into bud early and H. heterophyllus are now in full bloom on the nofthern end of the Sunshine Coast. Dion Harrison reports some early blooms from the Mount Crosby Cliffs and Kenmore near Brisbane. At Buderim our rainfall for June was 211mm, our average for that month being 14lmm. Unfortunately this coastal rain hasn't penetrated inland to much extent, where it is much needed in the dam catchment areas. H. -
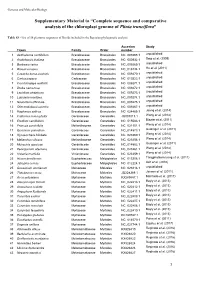
Complete Sequence and Comparative Analysis of the Chloroplast Genome of Plinia Trunciflora”
Genetics and Molecular Biology Supplementary Material to “Complete sequence and comparative analysis of the chloroplast genome of Plinia trunciflora” Table S3 - List of 56 plastome sequences of Rosids included in the Bayesian phylogenetic analysis. Accesion Study Taxon Family Order number 1 Aethionema cordifolium Brassicaceae Brassicales NC_009265.1 unpublished 2 Arabidopsis thaliana Brassicaceae Brassicales NC_000932.1 Sato et al. (1999) 3 Barbarea verna Brassicaceae Brassicales NC_009269.1 unpublished 4 Brassica napus Brassicaceae Brassicales NC_016734.1 Hu et al. (2011) 5 Capsella bursa-pastoris Brassicaceae Brassicales NC_009270.1 unpublished 6 Carica papaya Caricaceae Brassicales NC_010323.1 unpublished 7 Crucihimalaya wallichii Brassicaceae Brassicales NC_009271.1 unpublished 8 Draba nemorosa Brassicaceae Brassicales NC_009272.1 unpublished 9 Lepidium virginicum Brassicaceae Brassicales NC_009273.1 unpublished 10 Lobularia maritima Brassicaceae Brassicales NC_009274.1 unpublished 11 Nasturtium officinale Brassicaceae Brassicales NC_009275.1 unpublished 12 Olimarabidopsis pumila Brassicaceae Brassicales NC_009267.1 unpublished 13 Raphanus sativus Brassicaceae Brassicales NC_024469.1 Jeong et al. (2014) 14 California macrophylla Geraniaceae Geraniales JQ031013.1 Weng et al. (2014) 15 Erodium carvifolium Geraniaceae Geraniales NC_015083.1 Blazier et al. (2011) 16 Francoa sonchifolia Melianthaceae Geraniales NC_021101.1 Weng et al. (2014) 17 Geranium palmatum Geraniaceae Geraniales NC_014573.1 Guisinger et al. (2011) 18 Hypseocharis bilobate Geraniaceae Geraniales NC_023260.1 Weng et al. (2014) 19 Melianthus villosus Melianthaceae Geraniales NC_023256.1 Weng et al. (2014) 20 Monsonia speciose Geraniaceae Geraniales NC_014582.1 Guisinger et al. (2011) 21 Pelargonium alternans Geraniaceae Geraniales NC_023261.1 Weng et al. (2014) 22 Viviania marifolia Vivianiaceae Geraniales NC_023259.1 Weng et al. (2014) 23 Hevea brasiliensis Euphorbiaceae Malpighiales NC_015308.1 Tangphatsornruang et al. -

51729 ANBG Friends Nletter
Friends of the Australian National Botanic Gardens NEWSLETTER Number 60 November 2008 Lift Out: What‛s out in the Gardens & What‛s on at the Gardens A happier prospect Alan Munns, President, Friends of ANBG. At the time the last Newsletter went to press I was pessimistic about the Gardens budget for 2008-09. At that time we knew that Parks Australia had not done well in the budget and I worried that the Gardens might face yet more staff cuts. Things turned out better than I feared. The Gardens got a small increase in its budget which enabled some new staff to be engaged to help with review of the management plan, asset management and the education program. The Friends Patron Mrs Marlena Jeffery welcome this development, and will continue to press for more resources for the President Alan Munns Gardens. Vice President Barbara Podger Secretary David Coutts Friends’ springtime activities this year have been very popular. Treasurer Beverley Fisher Membership Secretary Barbara Scott We provided funds to support the creation of a Spring Public Officer David Coutts Display of flowering annual plants—daisies and Sturt’s General Committee Don Beer Desert Pea. Guided spring flower walks followed by morn- John Connolly ing tea at Hudsons Café were very popular. We hope this Les Fielke Louise Muir will become an annual event. Warwick Wright Activities Coordinator Warwick Wright Anne Rawson Photo by Social events: Louise Muir Breakfast with the Birds has attracted large numbers of Newsletter Committee Margaret Clarke visitors to early-morning guided walks led by enthusias- Barbara Podger Anne Rawson tic birdos, followed by breakfast at Hudsons Café. -

Gossypium Herbaceum and G
RESEARCH ARTICLE A New Synthetic Allotetraploid (A1A1G2G2) between Gossypium herbaceum and G. australe: Bridging for Simultaneously Transferring Favorable Genes from These Two Diploid Species into Upland Cotton Quan Liu☯, Yu Chen☯, Yu Chen¤, Yingying Wang, Jinjin Chen, Tianzhen Zhang, a11111 Baoliang Zhou* State Key Laboratory of Crop Genetics & Germplasm Enhancement, MOE Hybrid Cotton R&D Engineering Research Center, Nanjing Agricultural University, Nanjing, Jiangsu, People’s Republic of China ☯ These authors contributed equally to this work. ¤ Current address: Key Laboratory of Cotton Breeding and Cultivation in Huang-Huai-Hai Plain, Ministry of Agriculture, Cotton Research Center of Shandong Academy of Agricultural Sciences, Jinan, Shandong, OPEN ACCESS People’s Republic of China * [email protected] Citation: Liu Q, Chen Y, Chen Y, Wang Y, Chen J, Zhang T, et al. (2015) A New Synthetic Allotetraploid (A1A1G2G2) between Gossypium herbaceum and G. australe: Bridging for Simultaneously Transferring Abstract Favorable Genes from These Two Diploid Species into Upland Cotton. PLoS ONE 10(4): e0123209. Gossypium herbaceum, a cultivated diploid cotton species (2n = 2x = 26, A1A1), has favor- doi:10.1371/journal.pone.0123209 able traits such as excellent drought tolerance and resistance to sucking insects and leaf Academic Editor: Jean-Marc Lacape, CIRAD, curl virus. G. australe, a wild diploid cotton species (2n = 2x = 26, G2G2), possesses numer- FRANCE ous economically valuable characteristics such as delayed pigment gland morphogenesis Received: November 20, 2014 (which is conducive to the production of seeds with very low levels of gossypol as a potential Accepted: March 1, 2015 food source for humans and animals) and resistance to insects, wilt diseases and abiotic stress. -

Resistant Germplasm in Gossypium Species and Related Plants to the Reniform Nematode
Louisiana State University LSU Digital Commons LSU Historical Dissertations and Theses Graduate School 1981 Resistant Germplasm in Gossypium Species and Related Plants to the Reniform Nematode. Choi-pheng Yik Louisiana State University and Agricultural & Mechanical College Follow this and additional works at: https://digitalcommons.lsu.edu/gradschool_disstheses Recommended Citation Yik, Choi-pheng, "Resistant Germplasm in Gossypium Species and Related Plants to the Reniform Nematode." (1981). LSU Historical Dissertations and Theses. 3622. https://digitalcommons.lsu.edu/gradschool_disstheses/3622 This Dissertation is brought to you for free and open access by the Graduate School at LSU Digital Commons. It has been accepted for inclusion in LSU Historical Dissertations and Theses by an authorized administrator of LSU Digital Commons. For more information, please contact [email protected]. INFORMATION TO USERS This was produced from a copy of a document sent to us for microfilming. While the most advanced technological means to photograph and reproduce this document have been used, the quality is heavily dependent upon the quality of the material submitted. The following explanation of techniques is provided to help you understand markings or notations which may appear on this reproduction. 1.The sign or “target” for pages apparently lacking from the document photographed is “Missing Page(s)”. If it was possible to obtain the missing page(s) or section, they are spliced into the film along with adjacent pages. This may have necessitated cutting through an image and duplicating adjacent pages to assure you of complete continuity. 2. When an image on the film is obliterated with a round black mark it is an indication that the film inspector noticed either blurred copy because of movement during exposure, or duplicate copy. -

Native Plants for NSW V51 N2.Pdf
NativeNative PlantsPlants forfor NewNew SouthSouth WalesWales $5.00 www.austplants.com.au Journal of the Australian Plants Society NSWNative Ltd VolPlants 51 April No 2016 2 April — Page 2016 1 Native Plants for NSW Published quarterly in January, April, July and Contents October by the Australian Plants Society NSW Ltd ACN 002 680 408 Introduction ...................................... 3 Editor: David Crawford AGM and May gathering.................. 4 Proof Reading: Rhonda Daniels Coates Wildlife Tours .......................7 Jan Douglas Pink Flannel Flowers ....................... 8 Layout: Lachlan McLaine Report on February gathering ....... 10 The Journal is a forum for the exchange of views of members and others and their Book review ................................... 13 experiences of propagating, conserving and gardening with Australian plants. Inverawe Gardens ......................... 13 Contributions are warmly welcomed. They President’s report .......................... 14 may be emailed, typed or hand written and accompanied by photographs and drawings. If Conservation Report...................... 16 handwritten, please print botanical names and names of people. Fred Rogers Seminar .................... 17 2016 Get-together in Tamworth ..... 18 Photographs may be submitted as either high resolution digital ¿ les, such as jpg, or prints. Get-together registration form ....... 21 APS NSW Of¿ ce Mail: PO Box 5026 New members ............................... 22 Old Toongabbie NSW 2146 District Group directory ..................23 Phone: (02) 9631 4085 Email: of¿ [email protected] Membership form........................... 24 Website: www.austplants.com.au Facebook: www.facebook.com/APSNSW District Group directory continued . 26 Deadline for the July 2016 issue is Seed Bank Annual list.................... 27 1 June 2016. Acacia diphylla............................... 30 Deadline for the October 2016 issue is NSW South Coast 1 September 2016. -

Molecular Systematics in Gossypium and Its Relatives Tosak Seelanan Iowa State University
Iowa State University Capstones, Theses and Retrospective Theses and Dissertations Dissertations 1998 Molecular systematics in Gossypium and its relatives Tosak Seelanan Iowa State University Follow this and additional works at: https://lib.dr.iastate.edu/rtd Part of the Botany Commons Recommended Citation Seelanan, Tosak, "Molecular systematics in Gossypium and its relatives " (1998). Retrospective Theses and Dissertations. 11806. https://lib.dr.iastate.edu/rtd/11806 This Dissertation is brought to you for free and open access by the Iowa State University Capstones, Theses and Dissertations at Iowa State University Digital Repository. It has been accepted for inclusion in Retrospective Theses and Dissertations by an authorized administrator of Iowa State University Digital Repository. For more information, please contact [email protected]. INFORMATION TO USERS This manuscript has been reproduced from the microfilm master. UMI films the text directly from the original or copy submitted. Thus, some thesis and dissertation copies are in typewriter fece, \^e others may be from any type of computer printer. The quality of this reproduction is dependent upon the quality of the copy submitted. Broken or indistinct print, colored or poor quality illustrations and photographs, print bleedthrough, substandard margins, and improper alignment can adversely affect reproduction. In the unlikely event that the author did not send UMI a complete manuscript and there are missing pages, these will be noted. Also, if unauthorized copyright material had to be removed, a note will indicate the deletion. Oversize materials (e.g., maps, drawings, charts) are reproduced by sectioning the original, beginning at the upper lefr-hand comer and continuing from lefl to right in equal sections with small overlaps. -

Nuclear DNA Amounts in Angiosperms: Targets, Trends and Tomorrow
Annals of Botany Page 1 of 124 doi:10.1093/aob/mcq258, available online at www.aob.oxfordjournals.org Nuclear DNA amounts in angiosperms: targets, trends and tomorrow M. D. Bennett* and I. J. Leitch Jodrell Laboratory, Royal Botanic Gardens, Kew, Richmond, Surrey TW9 3AB, UK * For correspondence. E-mail: [email protected] Received: 25 August 2010 Returned for revision: 18 October 2010 Accepted: 24 November 2010 CONTENTS INTRODUCTION 2 Extending the range of genome sizes encountered in angiosperms 3 The need for reference lists 4 TARGETS IN GENOME SIZE RESEARCH 4 Downloaded from Meeting targets for species representation 4 Progress towards targets for familial representation 5 Improved systematic representation for genera 6 Improved representation of other groups 6 TRENDS IN TECHNIQUES USED TO ESTIMATE GENOME SIZE 7 The rise in flow cytometry as the technique of choice for genome size estimations 7 http://aob.oxfordjournals.org/ Development of different isolation buffers for flow cytometry 7 The application of flow cytometry to plant systematics 8 Recent developments in the application of flow cytometry to genome size studies 8 (i) The use of seeds 8 (ii) Ease of access to methodological data 8 (iii) New equipment 8 Are there any new techniques for estimating genome size on the horizon? 9 (i) Can real time PCR be used for estimating plant genome sizes? 9 (ii) Will ‘complete’ genome sequencing give useable genome size estimates? 9 TOMORROW 13 at NIH Library on December 30, 2015 Uncovering and collating genome size data from diverse published sources 14 Screening ex situ and in situ collections as sources of target taxa 15 DEDICATION 15 LITERATURE CITED 16 APPENDIX 19 Notes to the Appendix 19 Original references for DNA values 121 † Background and Aims The amount of DNA in an unreplicated gametic chromosome complement is known as the C-value and is a key biodiversity character of fundamental significance with many practical and predictive uses. -

The Complete Chloroplast Genome Sequence of Mahonia Bealei
Gene 528 (2013) 120–131 Contents lists available at ScienceDirect Gene journal homepage: www.elsevier.com/locate/gene The complete chloroplast genome sequence of Mahonia bealei (Berberidaceae) reveals a significant expansion of the inverted repeat and phylogenetic relationship with other angiosperms Ji Ma a, Bingxian Yang a, Wei Zhu a, Lianli Sun a, Jingkui Tian a,⁎, Xumin Wang b,⁎ a College of Biomedical Engineering & Instrument Science, Zhejiang University, 38 Zheda Road, Hangzhou, Zhejiang, China b Beijing Institute of Genomics, Chinese Academy of Science, Beijing, China article info abstract Article history: Mahonia bealei (Berberidaceae) is a frequently-used traditional Chinese medicinal plant with efficient anti- Accepted 18 July 2013 inflammatory ability. This plant is one of the sources of berberine, a new cholesterol-lowering drug with anti- Available online 27 July 2013 diabetic activity. We have sequenced the complete nucleotide sequence of the chloroplast (cp) genome of M. bealei. The complete cp genome of M. bealei is 164,792 bp in length, and has a typical structure with large Keywords: (LSC 73,052 bp) and small (SSC 18,591 bp) single-copy regions separated by a pair of inverted repeats (IRs Mahonia bealei 36,501 bp) of large size. The Mahonia cp genome contains 111 unique genes and 39 genes are duplicated in Chloroplast IR expansion the IR regions. The gene order and content of M. bealei are almost unarranged which is consistent with the SSRs hypothesis that large IRs stabilize cp genome and reduce gene loss-and-gain probabilities during evolutionary Molecular dating process. A large IR expansion of over 12 kb has occurred in M. -

Tree Planting Guide
Tree Planting Guide This guide is designed to help you with selecting and positioning trees to lessen the risk of roots blocking sewerage pipes. TREES AND THE HOME GARDEN What desolate places out cities and towns would be without trees in home gardens! Most of these trees are a real blessing. Unfortunately, though, a few of them are a nuisance, because they are unsuitable for their particular location. They can cause their owners a variety of serious problems, some of which are very expensive to fix. This booklet has been produced to help owners select tree and shrub species that will minimise the most common problem caused by trees in home gardens - chokes in sewerage pipes. The aim is certainly not to discourage people from planting trees - quite the opposite. Many people are very cautious and don’t plant trees anywhere near their sewerage pipes; this leaflet lists many species that they can confidently plant within a reasonable distance of their pipes - many as close as 2 metres. TREES AND SEWERAGE PIPES Fine hair roots will seek out and penetrate even very small cracks or joints in pipes. In addition the force exerted by larger roots can crack previously sound pipes, providing an avenue for root entry. Once roots have entered a pipe they grow in quite startling fashion, and can cause serious and costly failures in pipe systems. The biggest problem is with sewer pipes, but tree roots can cause problems with water reticulation and stormwater drainage pipes as well. Few people realise how aggressive the roots of some trees and shrubs can be in their relentless quest for water and nutrients.