Tetraethyl Pyrophosphate (TEPP)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Chemical Name Federal P Code CAS Registry Number Acutely
Acutely / Extremely Hazardous Waste List Federal P CAS Registry Acutely / Extremely Chemical Name Code Number Hazardous 4,7-Methano-1H-indene, 1,4,5,6,7,8,8-heptachloro-3a,4,7,7a-tetrahydro- P059 76-44-8 Acutely Hazardous 6,9-Methano-2,4,3-benzodioxathiepin, 6,7,8,9,10,10- hexachloro-1,5,5a,6,9,9a-hexahydro-, 3-oxide P050 115-29-7 Acutely Hazardous Methanimidamide, N,N-dimethyl-N'-[2-methyl-4-[[(methylamino)carbonyl]oxy]phenyl]- P197 17702-57-7 Acutely Hazardous 1-(o-Chlorophenyl)thiourea P026 5344-82-1 Acutely Hazardous 1-(o-Chlorophenyl)thiourea 5344-82-1 Extremely Hazardous 1,1,1-Trichloro-2, -bis(p-methoxyphenyl)ethane Extremely Hazardous 1,1a,2,2,3,3a,4,5,5,5a,5b,6-Dodecachlorooctahydro-1,3,4-metheno-1H-cyclobuta (cd) pentalene, Dechlorane Extremely Hazardous 1,1a,3,3a,4,5,5,5a,5b,6-Decachloro--octahydro-1,2,4-metheno-2H-cyclobuta (cd) pentalen-2- one, chlorecone Extremely Hazardous 1,1-Dimethylhydrazine 57-14-7 Extremely Hazardous 1,2,3,4,10,10-Hexachloro-6,7-epoxy-1,4,4,4a,5,6,7,8,8a-octahydro-1,4-endo-endo-5,8- dimethanonaph-thalene Extremely Hazardous 1,2,3-Propanetriol, trinitrate P081 55-63-0 Acutely Hazardous 1,2,3-Propanetriol, trinitrate 55-63-0 Extremely Hazardous 1,2,4,5,6,7,8,8-Octachloro-4,7-methano-3a,4,7,7a-tetra- hydro- indane Extremely Hazardous 1,2-Benzenediol, 4-[1-hydroxy-2-(methylamino)ethyl]- 51-43-4 Extremely Hazardous 1,2-Benzenediol, 4-[1-hydroxy-2-(methylamino)ethyl]-, P042 51-43-4 Acutely Hazardous 1,2-Dibromo-3-chloropropane 96-12-8 Extremely Hazardous 1,2-Propylenimine P067 75-55-8 Acutely Hazardous 1,2-Propylenimine 75-55-8 Extremely Hazardous 1,3,4,5,6,7,8,8-Octachloro-1,3,3a,4,7,7a-hexahydro-4,7-methanoisobenzofuran Extremely Hazardous 1,3-Dithiolane-2-carboxaldehyde, 2,4-dimethyl-, O- [(methylamino)-carbonyl]oxime 26419-73-8 Extremely Hazardous 1,3-Dithiolane-2-carboxaldehyde, 2,4-dimethyl-, O- [(methylamino)-carbonyl]oxime. -

Healthcare Resource
Health Care Resource: Links between Pesticide Exposures and Mental Health Prepared by the Mental Health and Environment Working Group of the Collaborative on Health and the Environment www.healthandenvironment.org Mental Health Effects from Pesticide Exposure Exposure to pesticides can have behavioral and psychiatric consequences. The purpose of this resource is to help mental health clinicians and health care providers become aware of the association between pesticide exposure and these consequences and to provide links to relevant research findings. Many people are at risk of pesticide exposure, including those who: • work in agriculture, landscaping or other settings (such as grocery stores, schools, daycares and office buildings) in which pest problems are treated with chemicals; • live downwind from where aerial spraying of pesticides is done; • live in communities where spray and runoff can contaminate both surface and ground water; or • live in urban dwellings where pest control is used. Outside use may take months for the pesticide to degrade to half-life or roughly half potency. Indoors there are no degrading elements such as sunshine, soil or rain to degrade potency; these undegraded pesticides can be re-suspended into the air in dust particles. Health care providers should consider the possibility that the symptoms associated with mental health disorders, such as irritability, depression or anxiety, may be the result of acute or chronic pesticide exposure. No matter what the presenting ailment may be, clinicians who care for individuals either at risk of pesticide exposure or those with known exposure are encouraged to inquire about the presence of depression, anxiety, or any of the other symptoms listed below. -

Environmental Health Criteria 63 ORGANOPHOSPHORUS
Environmental Health Criteria 63 ORGANOPHOSPHORUS INSECTICIDES: A GENERAL INTRODUCTION Please note that the layout and pagination of this web version are not identical with the printed version. Organophophorus insecticides: a general introduction (EHC 63, 1986) INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY ENVIRONMENTAL HEALTH CRITERIA 63 ORGANOPHOSPHORUS INSECTICIDES: A GENERAL INTRODUCTION This report contains the collective views of an international group of experts and does not necessarily represent the decisions or the stated policy of the United Nations Environment Programme, the International Labour Organisation, or the World Health Organization. Published under the joint sponsorship of the United Nations Environment Programme, the International Labour Organisation, and the World Health Organization World Health Orgnization Geneva, 1986 The International Programme on Chemical Safety (IPCS) is a joint venture of the United Nations Environment Programme, the International Labour Organisation, and the World Health Organization. The main objective of the IPCS is to carry out and disseminate evaluations of the effects of chemicals on human health and the quality of the environment. Supporting activities include the development of epidemiological, experimental laboratory, and risk-assessment methods that could produce internationally comparable results, and the development of manpower in the field of toxicology. Other activities carried out by the IPCS include the development of know-how for coping with chemical accidents, coordination -

Guide for the Selection of Personal Protective Equipment for Emergency First Responders
U.S. Department of Justice Office of Justice Programs National Institute of Justice National Institute of Justice Law Enforcement and Corrections Standards and Testing Program Guide for the Selection of Personal Protective Equipment for Emergency First Responders NIJ Guide 102–00 Volume I November 2002 U.S. Department of Justice Office of Justice Programs 810 Seventh Street N.W. Washington, DC 20531 John Ashcroft Attorney General Deborah J. Daniels Assistant Attorney General Sarah V. Hart Director, National Institute of Justice For grant and funding information, contact: Department of Justice Response Center 800–421–6770 Office of Justice Programs National Institute of Justice World Wide Web Site World Wide Web Site http://www.ojp.usdoj.gov http://www.ojp.usdoj.gov/nij U.S. Department of Justice Office of Justice Programs National Institute of Justice Guide for the Selection of Personal Protective Equipment for Emergency First Responders NIJ Guide 102-00, Volume I Dr. Alim A. Fatah1 John A. Barrett2 Richard D. Arcilesi, Jr.2 Charlotte H. Lattin2 Charles G. Janney2 Edward A. Blackman2 Coordination by: Office of Law Enforcement Standards National Institute of Standards and Technology Gaithersburg, MD 20899–8102 Prepared for: National Institute of Justice Office of Science and Technology Washington, DC 20531 November 2002 This document was prepared under CBIAC contract number SPO-900-94-D-0002 and Interagency Agreement M92361 between NIST and the Department of Defense Technical Information Center (DTIC). NCJ 191518 1National Institute of Standards and Technology, Office of Law Enforcement Standards. 2Battelle Memorial Institute. National Institute of Justice Sarah V. Hart Director This guide was prepared for the National Institute of Justice, U.S. -

View Is Primarily on Addressing the Issues of Non-Permanently Charged Reactivators and the Development of Treatments for Aged Ache
Design, Synthesis, and Evaluation of Therapeutics for the Treatment of Organophosphorus Poisoning by Nerve Agents and Pesticides Dissertation Presented in Partial Fulfillment of the Requirements for the Degree Doctor of Philosophy in the Graduate School of The Ohio State University By Andrew Joseph Franjesevic Graduate Program in Chemistry The Ohio State University 2019 Dissertation Committee Professor Christopher M. Hadad, Advisor Professor Thomas J. Magliery Professor David Nagib Professor Jonathan R. Parquette Copyrighted by Andrew Joseph Franjesevic 2019 2 Abstract Organophosphorus (OP) compounds, both pesticides and nerve agents, are some of the most lethal compounds known to man. Although highly regulated for both military and agricultural use in Western societies, these compounds have been implicated in hundreds of thousands of deaths annually, whether by accidental or intentional exposure through agricultural or terrorist uses. OP compounds inhibit the function of the enzyme acetylcholinesterase (AChE), and AChE is responsible for the hydrolysis of the neurotransmitter acetylcholine (ACh), and it is extremely well evolved for the task. Inhibition of AChE rapidly leads to accumulation of ACh in the synaptic junctions, resulting in a cholinergic crisis which, without intervention, leads to death. Approximately 70-80 years of research in the development, treatment, and understanding of OP compounds has resulted in only a handful of effective (and approved) therapeutics for the treatment of OP exposure. The search for more effective therapeutics is limited by at least three major problems: (1) there are no broad scope reactivators of OP-inhibited AChE; (2) current therapeutics are permanently positively charged and cannot cross the blood-brain barrier efficiently; and (3) current therapeutics are ineffective at treating the aged, or dealkylated, form of AChE that forms following inhibition of of AChE by various OPs. -

UNITED STATES PATENT OFFICE 2,572,806 MANUEFACTURE of TETRAETHY, PYROPHOSPAATE John Sterling Harris, Richmond Heights, Mo., Assignor to Monsanto Chemical Company, St
Patented Oct. 23, 1951 2,572,806 UNITED STATES PATENT OFFICE 2,572,806 MANUEFACTURE OF TETRAETHY, PYROPHOSPAATE John Sterling Harris, Richmond Heights, Mo., assignor to Monsanto Chemical Company, St. Louis, Mo., a corporation of Delaware No Drawing. Application May 1, 1948, Serial No. 24,683 2 Claims, (C. 260-461) 2 This invention relates to compositions con enty known to the art. A still further object of taining increased amounts of tetraethyl pyro this invention is to provide biological toxicant phosphate, and more. particularly to a process compositions containing increased amounts of for the manufacture of tetraethyl pyrophosphate. tetraethyl pyrophosphate which poSSeSS over Compositions containing tetraethyl pyrophos 5. S00% greater biological activity for the combat phate are widely used as agricultural economic ing and control of pests such as aphids and mites poisons, particularly against many insects such than do the compositions containing tetraethyl as aphids and against many acarina such as the pyrophosphate which are presently known to red Spider mites, however, such compositions may the art. be used generally against the lower forms of life O In the practice of this invention, the mixtures which, in the past, have been combatted by the of reaction products from the processes of this use of nicotine or nicotine salts. Furthermore, invention contain substantially 40%, that is tetraethyl pyrophosphate has been found useful 38-45%, of tetraethyl pyrophosphate as con in the preparation of insectivoricide and rodenti trasted with the processes of the prior art which cide compositions. 5 yielded mixtures of the reaction productS con While the art has disclosed several methods for taining only 10-15% of the tetraethyl pyrophoS the preparation of tetraethyl pyrophosphate, phate. -

NIH Public Access Provided by CDC Stacks Author Manuscript Chem Biol Interact
View metadata, citation and similar papers at core.ac.uk brought to you by CORE NIH Public Access provided by CDC Stacks Author Manuscript Chem Biol Interact. Author manuscript; available in PMC 2014 July 23. NIH-PA Author ManuscriptPublished NIH-PA Author Manuscript in final edited NIH-PA Author Manuscript form as: Chem Biol Interact. 2013 March 25; 203(1): 85–90. doi:10.1016/j.cbi.2012.10.019. Proteomic analysis of adducted butyrylcholinesterase for biomonitoring organophosphorus exposures Judit Marsillacha,b, Edward J. Hsiehb, Rebecca J. Richtera,b, Michael J. MacCossb, and Clement E. Furlonga,b,* Judit Marsillach: [email protected]; Edward J. Hsieh: [email protected]; Rebecca J. Richter: [email protected]; Michael J. MacCoss: [email protected]; Clement E. Furlong: [email protected] aDept. of Medicine (Division of Medical Genetics), University of Washington, 98195 Seattle, WA, USA bDept. of Genome Sciences, University of Washington, 98195 Seattle, WA, USA Abstract Organophosphorus (OP) compounds include a broad group of toxic chemicals such as insecticides, chemical warfare agents and antiwear agents. The liver cytochromes P450 bioactivate many OPs to potent inhibitors of serine hydrolases. Cholinesterases were the first OP targets discovered and are the most studied. They are used to monitor human exposures to OP compounds. However, the assay that is currently used has limitations. The mechanism of action of OP compounds is the inhibition of serine hydrolases by covalently modifying their active-site serine. After structural rearrangement, the complex OP inhibitor-enzyme is irreversible and will remain in circulation until the modified enzyme is degraded. Mass spectrometry is a sensitive technology for analyzing protein modifications, such as OP-adducted enzymes. -

Supplementary Material Modeling Chemical Interaction Profiles: I
Supplementary Material Modeling Chemical Interaction Profiles: I. Spectral Data-Activity Relationship and Structure-Activity Relationship Models for Inhibitors and Non-inhibitors of Cytochrome P450 CYP3A4 and CYP2D6 Isozymes Table 1. Tentative substrates and inhibitors of CYP3A4 isozyme. The calculations were carried out for hazardous waste chemicals recorded in the ATSDR EH Portfolio using the P450 substrate and P450 inhibitor modules of the ACD/ADME suite from Advanced Chemistry Development, Inc. (Toronto, ON, Canada). A probability (p) cutoff of 0.5 and reliability index (RI) cutoff of 0.4 were applied. Several compounds, whose activity was confirmed by screening the literature, are shown in bold (but an extensive systematic search for all compounds was not performed at this time). 3A4 CAS Substrates Inhibitors 3218-36-8 (1,1a-biphenyl)-4-carboxaldehyde 39001-02-0 1,2,3,4,6,7,8,9-octachlorodibenzofuran 67562-39-4 1,2,3,4,6,7,8-heptachlorodibenzofuran 35822-46-9 1,2,3,4,6,7,8-heptachlorodibenzo-p-dioxin 70648-25-8 1,2,3,4,6,7,9-heptachlorodibenzofuran 58200-70-7 1,2,3,4,6,7,9-heptachlorodibenzo-p-dioxin 69698-58-4 1,2,3,4,6,8,9-heptachlorodibenzofuran 55673-89-7 1,2,3,4,7,8,9-heptachlorodibenzofuran 38178-99-3 1,2,4,5,7,8-hexachoro-(9H)-xanthene 470-82-6 1,8-cineole 28548-08-5 12-ketoendrin 12-ketoendrin 132861-79-1 15-tetracosynoic acid, methyl ester 15-tetracosynoic acid, methyl ester 2597-11-7 1-hydroxychlordene 832-69-9 1-methylphenanthrene 2381-21-7 1-methylpyrene 5566-34-7 Gamma-chlordane Gamma-chlordane 2051-24-3 decachlorobiphenyl -

Organophosphate and Carbamate Reassessment the Costs and Benefits of Reassessing Carbaryl, Chlorpyrifos, and Diazinon (CDD)
Organophosphate and carbamate reassessment The costs and benefits of reassessing carbaryl, chlorpyrifos, and diazinon (CDD) NZIER report to the Environmental Protection Authority February 2015 About NZIER NZIER is a specialist consulting firm that uses applied economic research and analysis to provide a wide range of strategic advice to clients in the public and private sectors, throughout New Zealand and Australia, and further afield. NZIER is also known for its long-established Quarterly Survey of Business Opinion and Quarterly Predictions. Our aim is to be the premier centre of applied economic research in New Zealand. We pride ourselves on our reputation for independence and delivering quality analysis in the right form, and at the right time, for our clients. We ensure quality through teamwork on individual projects, critical review at internal seminars, and by peer review at various stages through a project by a senior staff member otherwise not involved in the project. Each year NZIER devotes resources to undertake and make freely available economic research and thinking aimed at promoting a better understanding of New Zealand’s important economic challenges. NZIER was established in 1958. Authorship This paper was prepared at NZIER by Chris Nixon. It was quality approved by Peter Clough. The assistance of Sarah Spring is gratefully acknowledged. L13 Grant Thornton House, 215 Lambton Quay | PO Box 3479, Wellington 6140 Tel +64 4 472 1880 | [email protected] © NZ Institute of Economic Research (Inc) 2012. Cover image © Dreamstime.com NZIER’s standard terms of engagement for contract research can be found at www.nzier.org.nz. While NZIER will use all reasonable endeavours in undertaking contract research and producing reports to ensure the information is as accurate as practicable, the Institute, its contributors, employees, and Board shall not be liable (whether in contract, tort (including negligence), equity or on any other basis) for any loss or damage sustained by any person relying on such work whatever the cause of such loss or damage. -

Organophosphorus Pesticide Air Monitoring Project
ORGANOPHOSPHORUS PESTICIDE AIR MONITORING PROJECT FINAL REPORT Submitted to Dr. Cynthia Lopez Washington State Department of Health Pesticide Program P.O. Box 47846 Olympia, WA 98504-7846 Prepared by Dr. Richard Fenske, Principal Investigator Dr. Michael Yost, Co-Principal Investigator Kit Galvin, CIH, Project Supervisor Maria Tchong, MPH, Research Industrial Hygienist Maria Negrete, Field Investigator Pablo Palmendez, MS, Field Investigator Cole Fitzpatrick, MS, Research Scientist Department of Environmental and Occupational Health Sciences University of Washington School of Public Health Box 357234 Seattle, WA 98195 Date June 30, 2009 ORGANOPHOSPHORUS PESTICIDE AIR MONITORING PROJECT FINAL REPORT Submitted to Dr. Cynthia Lopez Washington State Department of Health Pesticide Program P.O. Box 47846 Olympia, WA 98504-7846 Prepared by Dr. Richard Fenske, Principal Investigator Dr. Michael Yost, Co-Principal Investigator Kit Galvin, CIH, Project Supervisor Maria Tchong, MPH, Research Industrial Hygienist Maria Negrete, Field Investigator Pablo Palmendez, MS, Field Investigator Cole Fitzpatrick, MS, Research Scientist Department of Environmental and Occupational Health Sciences University of Washington School of Public Health Box 357234 Seattle, WA 98195 Date June 30, 2009 ACKNOWLEDGMENTS We gratefully acknowledge the contributions of Barbara Morrissey and Cynthia Lopez (Washington State Department of Health Pesticide Program) throughout the development and execution of this project. We also appreciate the service of the members of the project’s Technical Review Panel. The comments and suggestions provided by the panel have improved the quality of the project and this final report substantially. Dr Vince Hebert (Washington State University Food and Environmental Quality Laboratory) also provided very valuable comments and suggestions during the review process. -

Pdfs/Aum Chrn.Pdf
Air Force Institute of Technology AFIT Scholar Theses and Dissertations Student Graduate Works 3-21-2013 Biodegradation of an Organophosphate Chemical Warfare Agent Simulant by Activated Sludge with Varying Solid Retention Times Allen K. Janeczko Follow this and additional works at: https://scholar.afit.edu/etd Part of the Environmental Engineering Commons Recommended Citation Janeczko, Allen K., "Biodegradation of an Organophosphate Chemical Warfare Agent Simulant by Activated Sludge with Varying Solid Retention Times" (2013). Theses and Dissertations. 992. https://scholar.afit.edu/etd/992 This Thesis is brought to you for free and open access by the Student Graduate Works at AFIT Scholar. It has been accepted for inclusion in Theses and Dissertations by an authorized administrator of AFIT Scholar. For more information, please contact [email protected]. BIODEGRADATION OF AN ORGANOPHOSPHATE CHEMICAL WARFARE AGENT SIMULANT BY ACTIVATED SLUDGE WITH VARYING SOLID RETENTION TIMES THESIS Allen K. Janeczko, 2nd Lieutenant, USAF AFIT-ENV-13-M-38 DEPARTMENT OF THE AIR FORCE AIR UNIVERSITY AIR FORCE INSTITUTE OF TECHNOLOGY Wright-Patterson Air Force Base, Ohio DISTRIBUTION STATEMENT A APPROVED FOR PUBLIC RELEASE; DISTRIBUTION UNLIMITED The views expressed in this thesis are those of the author and do not reflect the official policy or position of the United States Air Force, Department of Defense, or the United States Government. AFIT-ENV-13-M-38 BIODEGRADATION OF AN ORGANOPHOSPHATE CHEMICAL WARFARE AGENT SIMULANT BY ACTIVATED SLUDGE WITH VARYING SOLID RETENTION TIMES THESIS Presented to the Faculty Department of Engineering Physics Graduate School of Engineering and Management Air Force Institute of Technology Air University Air Education and Training Command In Partial Fulfillment of the Requirements for the Degree of Master of Combating Weapons of Mass Destruction (Chemical Option) Allen K. -
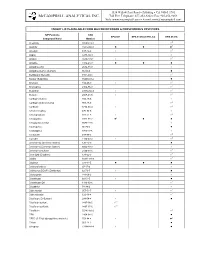
Compounds/Target Lists Detail
1534 Willow Pass Road • Pittsburg • CA 94565-1701 McCAMPBELL ANALYTICAL INC. Toll Free Telephone: 877-252-9262 • Fax: 925-252-9269 Web: www.mccampbell.com • E-mail: [email protected] TARGET LISTS AVAILABLE FROM MAI FOR NITROGEN & PHOSPHOROUS PESTICIDES N/P Pesticide CAS EPA 507 EPA 8141A (CTR List) EPA 8141A Compound Name Number Acephate 30560-19-1 zZ Alachlor 15972-60-8 z z zZ Ametryn 834-12-8 z zZ Aspon 3244-90-4 z Atraton 1610-17-9 z zZ Atrazine 1912-24-9 z z z Azinphos ethyl 2642-71-9 z Azinphos methyl (Guthion) 86-50-0 z Benfluralin (Benefin) 1861-40-1 zZ Bolstar (Sulprofos) 35400-43-2 z Bromacil 314-40-9 z zZ Bromophos 2104-96-3 zZ Butachlor 23184-66-9 z zZ Butylate 2008-41-5 z zZ Carbophenothion 786-19-6 z Carbophenothion methyl 953-17-3 zZ Carboxin 5234-68-4 z zZ Chlorfenvinphos 470-90-6 z Chloropropham 101-21-3 z zZ Chlorpyrifos 2921-88-2 zZ z z Chlorpyrifos methyl 5598-13-0 z Coumaphos 56-72-4 z Crotoxyphos 7700-17-6 z Crufomate 299-86-5 zZ Cycloate 1134-23-2 z zZ Demeton-O (Demeton isomer) 126-75-0 z Demeton-S (Demeton isomer) 8065-48-3 z Demeton-S sulfone 2496-91-5 zZ Deet (Off) (Dephere) 134-62-3 zZ Dialifor 10311-84-9 zZ Diazinon 333-41-5 z z z Dichlorofenthion 97-17-6 z Dichlorvos (DDVP) (Dichlorfos) 62-73-7 z z Dicrotophos 141-66-2 z Dimethoate 60-51-5 z z Dimethoate OA 1113-02-6 zZ Dioxathion 78-34-2 z Diphenamid 957-51-7 z zZ Diphenylamine 122-39-4 zZ Disulfoton (Di-Syston) 298-04-4 z z Disulfoton sulfone 2497-06-5 zX1 Disulfoton sulfoxide 2497-07-6 zX1 Ethalflurin 55283-68-6 zZ EPN 2104-64-5 z EPTC (S-Ethyl dipropylthiocarbamate) 759-94-4 z zZ Ethion 563-12-2 z Ethoprop 13194-48-4 z z 1534 Willow Pass Road • Pittsburg • CA 94565-1701 McCAMPBELL ANALYTICAL INC.