Stability and Availability Of
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Heirloom Tomato Varieties Available at the 2105 Great Tomato Plant Sale
CONTRA COSTA MASTER GARDENERS UNIVERSITY OF CALIFORNIA COOPERATIVE EXTENSION ccmg.ucanr.edu April 2105 Heirloom Tomato Varieties Available at the 2105 Great Tomato Plant Sale April 4, 10 am –3 pm at Our Garden –and– April 11, 10 am – 2 pm at Our Garden & in Richmond(+Rch) For purposes of propagation, Contra Costa Master Gardeners use only seeds from open‐ pollinated varieties that breed true. We have collected many of these seeds ourselves from our own gardens (MG Seed Stock). We do not use any GMO seeds. Quotes in italics are from Contra Costa Master Gardeners! Number of days from transplant to fruit ripening is approximate and will vary with environmental & cultural conditions. Amish Paste (Indeterminate—85 days). From Lancaster, PA. At 8‐12 ounces, it’s one of the largest sauce tomatoes and has superior flavor. Vigorous vines bear heavily over a long season. Flesh is juicy and meaty, excellent for sauce, canning or fresh eating. Tolerant of cooler summers. (+Rch) . Ananas Noire‐organic (Indeterminate—80 days). Back by popular demand! Last year, customers asked again and again for this classic Belgian tomato. Yellow beefsteak with red blush can be 1 to 1½ lbs! Great sweet, lip‐smacking taste with a hint of acidity. (“Black Pineapple" with a distinctive, streaked interior of pink, red, green and yellow. Large and sprawling plant with large, green‐shouldered, dark purple fruits. The fruits have a complex, sweet and rich taste. Makes a great tomato sauce”.) Returning in 2015! . Aunt Ruby’s German Green (Indeterminate—78 days). Beefsteak from “Aunt Ruby” of Greeneville, Tennessee. -

Variety Guide: 2021
Variety Guide: 2021 A note on where we source our seeds We are fortunate to have a long-standing relationship with Johnny’s Selected Seeds, an employee-owned company based out of Maine. All of the seeds we get in bulk to redistribute to you at Winter Meeting and many of the seeds we grow into seedlings come from Johnny’s. They specialize in plant breeding (non-GMO), research, and seed production using both conventional and organic methods. A number of the varieties we provide are actually developed by Johnny’s – they will be marked in this guide as “Bred by Johnny’s”. Johnny’s has a wealth of helpful educational resources in their Grower’s Library, and you can type variety names into the search bar on their website to find more growing information. This year we are excited to source a significant number of varieties from local seed company Truelove Seeds. Truelove offers rare, open pollinated, and culturally important vegetable, herb, and flower seeds grown by more than 20 small-scale urban and rural farmers committed to community food sovereignty, cultural preservation, and sustainable agriculture – many of them are based in Philadelphia and the surrounding area. Through this collaboration, growers share their own seeds and stories and bring in extra financial support for the important work they do building community sovereignty through agroecology. Scrolling through Truelove’s online catalogue is an incredible experience because along with growing and harvesting information, many variety descriptions include stories behind the seeds, cooking recommendations, and growers’ sentiments on their relationship to the plants. -

Fresh Bright Harvest
2020 VEGETABLE & HERB COLLECTION TM fresh ideas harvest bright fresh HANDPICKED VEGETABLES & HERBS / VARIETY NAME PEPPER-MANIA! Gardeners and cooks want an array of flavors and sizes…you can deliver. Our NEW Orange Marmalade Sweet Bell Pepper brings a pop of bright, fresh color to the table. Plus, it tastes as sweet as it looks! p 9. TASTIEST TOMATOES! Meet the Nothing compares to a HandPicked sun-warmed tomato fresh off the vine. All you need is Collection one bite of our super-sweet, craveable NEW Artemis PanAmerican Seed offers Cherry Tomato! Find it with a novel, high-quality group our full tomato collection starting on p 10. of vegetables and herbs especially for those who supply fresh market farmers and hobby gardeners. What sets HandPicked varieties apart are their fresh, home-grown flavor… exceptional fruit quality…earlier and longer harvests…loads of produce…and many feature compact habits to fit smaller BRILLIANT BASIL! growing spaces. Our basils offer excellent performance in multiple growing environments. Each offers something special, like innovation in disease resistance, long flowering and savory flavors. For large leaves, sweet flavor and high resistance to Fusarium, check out NEW Newton Basil on p 17. 2 Find product & growing info at panamseed.com/HandPicked HANDPICKED VEGETABLES & HERBS / CUCUMBER Martini CUCUMBER Cucumis sativus Seed supplied as: Raw Gherking Patio Snacker Name Type Fruit Days to Fruit size Flower type Disease Comments characteristics maturity resistance from direct seed Gherking F1 European Medium green 55-60 3-5 in./ Gynoecious, HR: Early, large yields of bitter-free fruit; won’t pickler skin, highly 8-13 cm long parthenocarpic CMV-1, S become bitter if left on the vine. -
PANINI PASTA Add to Any Pasta: PIATTI LUNCH
PASTA ASSORTED ARTISAN SALUMI SAMPLER 9 or Family Portion 17 SPAGHETTI AND MEATBALLS* 14 PROSCIUTTO DI PARMA House made meatballs, spicy plum tomato sauce Imported from Langhirianese, Italy, aged 16 months, sweet and buttery SHRIMP FRA DIAVOLO* 16 SPECK LUNCH Fettuccine, fiery plum tomato sauce, extra virgin olive oil Imported prosciutto from Balzano, Italy, air dry aged 10 months, cold smoked BOMBOLOTTI AI MODO MAX* 15 CAPOCOLLO Baked short rigatoni, hot and sweet Italian sausage, Delicate flavor, tender, rich texture, aged 6 months peas, plum tomatoes, grana parmesan cream SOPRESSATA STONE PIES PENNE ALLA VODKA 13 Handcrafted country style dried salami, aged up to 12 weeks, sharp, peppery finish Sun dried tomatoes, garlic, fresh basil, Add marinated mushrooms, long stem artichokes, roasted peppers, Grana Padano~2 each MAX’S RONI 16 Small crisp pepperoni, fresh and shredded mozzarella, parmesan, oregano San Marzano tomato vodka cream, aged parmesan LONG RIGATONI BOLOGNESE 15 CLASSIC MARGARITA 15 San Marzano tomatoes, buffalo mozzarella, sweet basil, Hearty Italian meat sauce, pesto, creamy ricotta PRIMI extra virgin olive oil, sea salt MAX A PENNE* 14 CALAMARI FRITTI 11 POLLO ALLA GENOVESE* 16 Wood grilled chicken, escarole, plum tomatoes, Crispy fried calamari, cherry peppers, family portion 20 Rosemary marinated chicken, caramelized onions, asiago, extra virgin olive oil, grana parmigiano lemon herb aioli, San Marzano tomato sauce gorgonzola, toasted pine nuts CONCHIGLIONI VERDURA 13 SICILIAN CALAMARI 11 TOMATO & PANCETTA 16 Local yellow and green squash, heirloom tomatoes , kalamata olives, Sauteed Point Judith calamari, kalamata olives, capers, anchovies, Shaved summer squash, Italian cured bacon, Kumato tomato, basil, lemon herb butter San Marzano tomatoes, garlic crostini imported fontina cheese, Grana Padano, evoo, fresh oregano Available over fresh linguine as an entree 15 La Veneziane gluten free corn pasta or whole wheat pasta available upon request. -

RHS AWARD of GARDEN MERIT Tomatoes 2011
RHS AWARD OF GARDEN MERIT Tomatoes 2011 The AGM criteria TOMATO (Solanum lycopersicum) Beefsteak tomato 97 ‘Alicante’ (H2) 03 ‘Amana Orange’ (H2) Intended as a practical guide for ~ good shape; heavy crop of ~ attractive orange-yellow fruit; solid the gardener, the AGM is awarded attractive fruits which ripen well flesh; very little seed. Good flavour only to plants that are: 97 ‘Arasta’ (H2) 03 ‘Beefsteak’ (H2) ~ F1 hybrid. Good shape and flavour; ~ very high yield; bright red, • excellent for ordinary use in very bright juicy flesh attractive, large fruits with solid appropriate conditions 97 ‘Cristal’ (H2) flesh and good flavour • available in the trade ~ F1 hybrid. Good shape and flavour; 03 ‘Costoluto Fiorentino’ (H2) • of good constitution very high yield of large, attractive ~ high yield; medium sized, • essentially stable in form and fruits with glossy skin and dark flesh attractive bright red, highly colour 97 ‘Golden Sunrise’ (H2) ribbed, succulent fruit with good • reasonably resistant to pests and ~ later-maturing; small yellow fruits flavour diseases 93 ‘Outdoor Girl’ (H2) 03 ‘Flame’ (H2) ~ early. Indeterminate, round red ~ low number of very large, AGM hardiness ratings fruits with good flavour attractive fruit; yellow flesh 97 ‘Pannovy’ (H2) merging into a flame-red core. Each award includes a hardiness ~ F1 hybrid. Thick skin; very high yield Very good flavour rating, which is an integral part of 97 ‘Piranto’ (H2) 03 ‘Legend’ (H2) the AGM, and should be included ~ F1 hybrid. Good shape and colour; ~ F1 hybrid; early. Determinate in any citation of the award: high yield of large fruits with good plants; large, bright red fruit. -
![[ Plug + Play ] Programs](https://docslib.b-cdn.net/cover/1136/plug-play-programs-1371136.webp)
[ Plug + Play ] Programs
plug + play [ VARIETY CATALOG 2011-2012 ] ® PLUG CONNECTION 2627 Ramona Drive Vista, California 92084 760.631.0992 760.940.1555 (fax) [email protected] plugconnection.com © 2011 PLUG CONNECTION. ALL RIGHTS RESERVED. PLUG INTO THE MOST STIMULATING SOURCE FOR DAZZLING PLANTS. OUR TEAM IS INTERLINKED WITH THE MOST FORWARD-THINKING BREEDERS ON THE PLANET, ALLOWING US TO BRING YOU IMPRESSIVE NEW VARIETIES AND TURN UP THE VOLUME ON THE CLASSICS. EACH OF THESE POWERFUL PLANTS COMES PACKAGED WITH SIMPLICITY, SERVICE AND SUPPORT, MAKING IT EASY TO KEEP YOUR BUSINESS SHOOTING UPWARD. INSTALL OUR PRODUCTS AND GET PROFITS. PROSPERITY. PEACE OF MIND. IF YOU’RE READY FOR THE GOOD STUFF, IT’S TIME TO HIT PLAY. PLUG + PLAY PROGRAMS. 17 WESTFLOWERS® BY WESTHOFF. 78 ERYSIMUM GLOW™. 18 ASTERS KICKIN™ . 82 contents ERYSIMUM RYSI. 19 BIDENS. 84 of ERYSIMUM POEM . 20 BRACTEANTHA . 84 ERYSIMUM WINTER. 21 COSMOS CHOCAMOCHA. 84 table BUDDLEJA BUZZ™ BUTTERFLY BUSH. 22 CHRYSOCEPHALUM. 85 GERANIUM FIREWORKS® COLLECTION. 24 OSTEOSPERMUM . 85 GERANIUM – IVY P. PELTATUM HYBRIDS . 25 LUCKY LANTERN™ ABUTILON . 85 GERANIUM – CRISPUM ANGEL EYES® SERIES. 26 ORGANIKS®. 86 GERANIUM – GRANDIFLORA ARISTO® SERIES. 27 TOMACCIO™. 92 GERANIUM ZONAL . 28 SUPERNATURALS™ GRAFTED VEGETABLES . 94 ANGELONIA PAC ADESSA® SERIES. 30 BAMBOO FROM TISSUE CULTURE. 98 BEGONIA SUMMERWINGS™ . 32 KIA ORA FLORA. 101 BEGONIA BELLECONIA™ . 33 COPROSMA. 102 TROPICAL SURGE. 34 HEBE . 104 DRAKENSBERG™ DAISY HARDY GARDEN GERBERA. 36 CORDYLINE . 104 BELARINA™ DOUBLE-FLOWERED PRIMULA. 38 ITOH PEONY. 106 NESSIE™ PLUS NEMESIA. 40 TECOMA BELLS OF FIRE™ . 108 KAROO™ NEMESIA. 41 TECOMA LYDIA™. 109 DIASCIA MARSHMALLOW™ SERIES. 42 POWERFUL PROGRAMS. 111 ALLURE™ OXALIS TRIANGULARIS HYBRIDS. -

Animal Aid's Plant Sale
ANIMAL AID’S PLANT SALE Saturday, May 9, 2020 by appointment only! Southwest Auto Body 4200 SW Beaverton-Hillsdale Highway $3.50/plant or 3 for $10 PINK TOMATOES CASPIAN PINK- Prolific 1-2 pound pink-red beefsteak tomatoes that rivals Brandywine for flavor. Does well in cooler climates. DANIELS- Large pink beefsteaks 8-17 oz., very few seeds excellent sweet flavor. MOMOTORO- Japanese hybrid, one of the most perfect tomatoes. Pink fruits, with a sweet taste. Early 60 days. MUSHROOM BASKET- Uniquely pleated fruits are firm with few seeds and sweet flavor. Heavy yield of 8-16 ounce pink fruits. ROZOVOYI KILOGRAMMOVY- Russian heirloom from Khilenko family. Huge pink beefsteak 10-26 oz. very juicy and meaty with a delicious sweet flavor. WATERMELON BEEFSTEAK- High yields of 1 to 2 pound pink beefsteak tomatoes. Very sweet, meaty, and juicy with old fashioned tomato flavor. RED TOMATOES BOBCAT F1-Hybrid, 68 days. Perfect smooth skin fruit, firm, and meaty red flesh. 10 ounce slicers load heavily on strong determinate plants. FANTASTIC- F1 Hybrid 3 to 5 inch round firm slicers. Fantastic’s meaty, bright red fruit are rich in beefsteak flavor. Very high yielding. SLAVA- Early 65 days, heavy producer of 1 to 2 ounce fruit with delicious flavor. SICILIAN SAUCER- Italian heirloom beefsteak type. Excellent production on semi determinate plants. They are very sweet, meaty and juicy. STUPICE- Early cold tolerant plants that produce great red salad tomatoes. 10 FINGERS OF NAPLES- Excellent paste type. 5 to 6 inch meaty fruit up to 6 ounces. Flavor is sweet, rich and superior to many modern varieties. -

Casey's Heirloom Tomatoes of Airdrie
Casey’s Heirloom Tomatoes of Airdrie Seeds for 2006 Jeffrey Casey [email protected] Casey’s Heirloom Tomatoes of Airdrie page 1/22 Introduction It is with great pleasure that I offer the following heirloom tomato seed catalogue for your perusal. My love for growing vegetables has grown exponentially from my first miniscule plot out behind my teachers’ housing unit in a small town in Hokkaido, Japan to my current garden in which I grew 85 different tomato varieties last year. Growing heirloom tomatoes has turned me into a passionate hobbyist so much so that I firmly believe that others should also grow them and this provided the genesis for this catalogue. The exact definition of an heirloom tomato may evoke controversy amongst some growers but I prefer to think of a heirloom as “something of value that can be passed on to the next generation”. Our forefathers practised diligently the time honoured tradition of saving the seed from the harvest to be planted again the following year. It is precisely because someone took the time to save the seed A single days’ harvest for a variety they thought was delicious enough to grow the following year that I am able to offer a wide range of heirloom tomatoes in terms of shapes, sizes, colours, flavours and textures. I have tried my utmost to research the history of each variety and provide it for you, however, some varietal histories are unfortunately lost in time. I have done my utmost to ensure the purity of my seeds that I offer by bagging the blossoms and fermenting the seeds. -
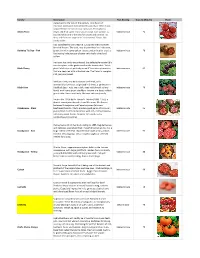
2021 Tomatoes
Variety Description Fruit Bearing Days to Maturity Photo Dates back to the turn of the century, Tom Hauch of Heirloom Seeds commercialized this variety in 1990. It was acquired from the Amish near Lancaster, Pennsylvania. Amish Paste Bright red 8-12 ounce fruits vary in shape from oxheart to Indeterminate 74 rounded plum and is the best for sauces and canning. Its deep red fruits are large with "real tomato" flavor. Not overly acidic. Big, succulent fruit can reach 8–12 ounces with a complex blend of flavors. The vivid, rosy-blushed fruit has iridescent, Berkeley Tie Dye - Pink green skin with sunny yellow streaks and an interior that’s a Indeterminate 70 fascinating kaleidoscope of green with trails of red and yellow. Heirloom too rarely encountered, this delectable variety fully merits a place in the garden and on the dinner table. Yields Black Cherry plentiful clusters of perfectly round 1" true cherry tomatoes Indeterminate 65 that are deep red with a blackish hue. The flavor is complex, rich, juicy and sweet. Medium-sized, very dark maroon beefsteak, with wonderfully rich flavor, originated in Crimea, a peninsula in Black Krim the Black Sea. Fruits are a dark, deep red (almost a shiny Indeterminate 70 black) with heavy green shoulders. Interior is a deep, reddish- green color. Sweet and tasty. Matures extremely early. Bred in the 1920s by Dr. Harold E. Martin (1888 -1959), a dentist turned plant breeder from Westown, PA. A cross between Brandywine and Fejee Improved (a brown Brandywine - Black beefsteak) tomato. Plants produce good yields of 10-16 oz., Indeterminate 95 purple/black beefsteak tomatoes with rich, earthy complex, tomatoey sweet flavors. -

2021 Grow Pittsburgh Plant Sale Catalog
2021 Plant Sale Herbs, Tomatoes, Flowers, Spring, Summer & Fall Vegetables New 2021 Varieties in Red! Please note: availability varies by location. Please check our website & social media for most up to date information. East End Food Co-op: Late March - June Garden Dreams: TBD Mother’s Day- July 17th Daily, 8 am - 8 pm Thursday & Friday 11:30 am - 6 pm 7516 Meade St, Pittsburgh, PA 15208 Saturday, 10 am - 4 pm 806 Holland Ave, Pittsburgh PA 15221 Annual Flowers (4 packs) Notes Type Description Majestic 4" blooms in tangerine orange, rich gold, and lemony yellow. They make radiant Crackerjack Marigold Yellow and Orange long-lasting cut flowers and are easy to grow . Queen Sophia French Marigold Orange Edible 2-3” blooms are orange with yellow edges on 10-12” plants. Attracts beneficial insects. Tangerine Gem Marigold Orange Edible Tiny, fragrant, deep orange flowers. Delicious citrus taste. Long-blooming, compact plants. Large, double blossoms approximately 4–5" in diameter, in multiple, magnificent colors. Benary's Giant Zinnias Pink, Orange, Yellow Edible Vigorous all-season producers; holds up well in summer rain and heat. Red, Orange, Attractive, fully-double and semi double blooms are 1-2" in size. Bright, earthy tones; Jazzy Mix Zinnias Edible Yellow Bi-color blooms Yellow, Bronze, Bold, beautiful flowers up to 8" across on 6-8’ multi-branching stems.. Bright yellow, Autumn Beauty Sunflower Purple bronze, and purple shades with some bi-colors. Dwarf, branching sunflower looks great in small beds and containers. 3-6" blooms are Big Smile Sunflower Yellow bright golden-yellow with a black center. -

2020 Tomatoes CHERRY Blue Cream Berries Chocolate Cherry a Super-Sweet Cherry Tomato, Cream Berries Are Plants Are Loaded with Clusters of Sweet and Super Prolific
509.928.3342 www.facebook.com/thetomatolady www.thetomatolady.com my blog: thetomatoladyblog.com [email protected] 2020 Tomatoes CHERRY Blue Cream Berries Chocolate Cherry A super-sweet cherry tomato, cream berries are Plants are loaded with clusters of sweet and super prolific. These boast a delicate but complex delicious little gems. The 1” cherry-like fruits are a flavor and a beautiful cream color with purple-blue rich brick red with deep chocolate shading. These splashes on the shoulder. They tend to crack easily are perfect for salads, with an exceptional fresh so I'd just pop them in my mouth as I picked. A Wild flavor that is rich and complex. Indeterminate, mid- Boar variety. Indeterminate, mid season\ Bumblebee Black Cherry - PF A spectacular mix of pink with yellow stripes, yellow Love the flavor, love how sweet it is, love the with red stripes and purple striped with metallic color, love how healthy the plant is! You will green. These are really pretty. Round, 1".crack- adore this blackish-purple skinned, cherry tomato resistant fruits. with dark red flesh and a sweet and juicy flavor. Indeterminate, mid Indeterminate, mid Chocolate Cherry Brown Berry Loaded with clusters of sweet little gems. The 1” Heirloom tomato produce vigorous regular-leaf cherry-like fruits are a rich brick red. Perfect for tomato plants that yield really large crops of salads with an exceptional flavor that is rich and mahogany (brick-red), 1” round, cherry tomatoes. complex. The Brown Berry cherry tomato has excellent semi- Indeterminate, mid sweet, fruity, with just a slight bit of an acid finish. -

Slicing Tomato Varieties
2017 Tomato Variety List The Henrys’ Plant Farm Indeterminate: Tomato varieties that should be staked, trellised, or caged for best results. Tomato varieties blossom and set tomatoes until plants are killed by a freeze. Determinate: Tomato varieties that are space-saving or bush type in the garden. They may be grown with or without support. The tomatoes ripen within a concentrated time period. Consider some varieties of this type if you want a supply of ample amounts of tomatoes for canning. Slicing Tomato Varieties Beefmaster Hybrid VFNASt—(80 Days) Indeterminate. Popular hybrid beefsteak type, good disease resistance, solid, meaty tomatoes up to 2#. Resists cracking. Beefy Boy Hybrid VFFTSt—(70 Days) Indeterminate. Meaty, extra-large main-season beefsteak type with a great taste. Solid 12-16 oz. deep red tomatoes. Heavy yields. KSU recommended. Better Boy Hybrid VFNASt—(75 Days) Indeterminate. Great midseason variety, deep red tomatoes up to 1#. Juicy, yet firm tomatoes. Excellent disease resistance. Better Bush VFFNASt—(68 Days) Indeterminate Short Internode. Compact, bushy growth habit. Produces 4” tomatoes weighing 8-10 oz. Excellent choice for large containers or the small garden. Big Beef Hybrid VFFNTASt—(73 Days) Indeterminate. Old fashioned rich flavor with bred-in hybrid qualities of large tomato size, good yields, uniformity and good disease resistance. Burpee’s Big Boy—(78 Days) Indeterminate. Large, crack-free tomatoes with absolute superb flavor. Bright red, firm, meaty tomatoes grow to 1# or more in size. Bush Early Girl Hybrid VFFNT—(54 Days) Determinate. Compact, patio-sized variety. Produces huge yields of firm, meaty, flavorful tomatoes. Bushsteak Hybrid—(65 Days) Determinate.