Lime (Citrus Aurantifolia (Christm.) Swingle) Essential Oils: Volatile Compounds, Antioxidant Capacity, and Hypolipidemic Effect
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
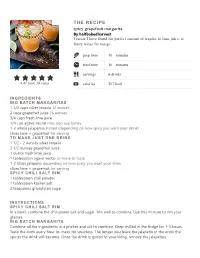
Spicy Grapefruit Margarita. the RECIPE
THE RECIPE spicy grapefruit margarita. By halfbakedharvest I swear I have found the perfect amount of tequila, to lime juice, to fruity mixer for margs prep time 10 minutes total time 10 minutes servings 6 drinks 4.47 from 39 votes calories 207 kcal INGREDIENTS BIG BATCH MARGARITAS 1 1/2 cups silver tequila 12 ounces 2 cups grapefruit juice 16 ounces 3/4 cups fresh lime juice 1/4 cup agave nectar may also use honey 1-2 whole jalapeños halved (depending on how spicy you want your drink) slices lime + grapefruit for serving TO MAKE JUST ONE DRINK 1 1/2 - 2 ounces silver tequila 2 1/2 ounces grapefruit juice 1 ounce fresh lime juice 1 tablespoon agave nectar or more to taste 1-2 slices jalapeno depending on how spicy you want your drink slices lime + grapefruit for serving SPICY CHILI SALT RIM 1 tablespoon chili powder 1 tablespoon kosher salt 2 teaspoons granulated sugar INSTRUCTIONS SPICY CHILI SALT RIM In a bowl, combine the chili power salt and sugar. Mix well to combine. Use this mixture to rim your glasses. BIG BATCH MARGARITA Combine all the ingredients in a pitcher and stir to combine. Keep chilled in the fridge for 1-3 hours. Taste the drink every hour to check for spiciness. The longer you leave the jalapeño in the drink the spicier the drink will become. Once the drink is spiced to your liking, remove the jalapeños. When ready to serve, salt the rims of 4-6 glasses. Add ice to each glass and pour the margaritas over the ice. -

Citrus Varieties in Egypt: an Impression
International Research Journal of Applied Sciences Short Communication pISSN: 2663-5577, eISSN: 2663-5585 Citrus Varieties in Egypt: An Impression Waleed Fouad Abobatta Department of Citrus, Horticulture Research Institute, Agriculture Research Center, Egypt ARTICLE INFORMATION ABSTRACT Received: Citrus industry is very important for Egyptian economy, citrus fruit is the leading exportable agricultural product of Egypt and is an important source of national income. Citrus cultivation Accepted: area represents about 29% of the total fruit area, there are different citrus varieties cultivated in Egypt. This work aims to provide a short description of main citrus varieties cultivated in Published: Egypt through providing information about fruit size, maturity periods, seediness and productivity average and main cultivated areas for each variety. However Washington Navel Corresponding Author: and Valencia orange are the main varieties followed by Mandarins group varieties, lemon, Waleed Fouad Abobatta, Balady orange, while other varieties like Grapefruit, Sour orange and Kumquat are cultivated Department of Citrus, in small areas. Horticulture Research Institute, Agriculture Research Center, Egypt Key words: Citrus industry, navel orange, valencia orange, mandarins group, citrus varieties INTRODUCTION Citrus is a genus from Rutaceae family, subfamily Aurantoideae1 and there are several species in this genus; but there are major species such as sweet orange (Citrus sinensis (L.) Osbeck), mandarins group, grapefruits (Citrus paradisi ), lime (Citrus aurantifolia) and sour orange (Citrus aurantium L.)2. Citrus is a diploid genus origin in tropical, subtropical, but now it is produced mainly in arid and semiarid regions. Citrus species are among the most widely grown fruit crops in the world and have a huge market all over the world3. -

Citrus from Seed?
Which citrus fruits will come true to type Orogrande, Tomatera, Fina, Nour, Hernandina, Clementard.) from seed? Ellendale Tom McClendon writes in Hardy Citrus Encore for the South East: Fortune Fremont (50% monoembryonic) “Most common citrus such as oranges, Temple grapefruit, lemons and most mandarins Ugli Umatilla are polyembryonic and will come true to Wilking type. Because most citrus have this trait, Highly polyembryonic citrus types : will mostly hybridization can be very difficult to produce nucellar polyembryonic seeds that will grow true to type. achieve…. This unique characteristic Citrus × aurantiifolia Mexican lime (Key lime, West allows amateurs to grow citrus from seed, Indian lime) something you can’t do with, say, Citrus × insitorum (×Citroncirus webberii) Citranges, such as Rusk, Troyer etc. apples.” [12*] Citrus × jambhiri ‘Rough lemon’, ‘Rangpur’ lime, ‘Otaheite’ lime Monoembryonic (don’t come true) Citrus × limettioides Palestine lime (Indian sweet lime) Citrus × microcarpa ‘Calamondin’ Meyer Lemon Citrus × paradisi Grapefruit (Marsh, Star Ruby, Nagami Kumquat Redblush, Chironja, Smooth Flat Seville) Marumi Kumquat Citrus × sinensis Sweet oranges (Blonde, navel and Pummelos blood oranges) Temple Tangor Citrus amblycarpa 'Nasnaran' mandarin Clementine Mandarin Citrus depressa ‘Shekwasha’ mandarin Citrus karna ‘Karna’, ‘Khatta’ Poncirus Trifoliata Citrus kinokuni ‘Kishu mandarin’ Citrus lycopersicaeformis ‘Kokni’ or ‘Monkey mandarin’ Polyembryonic (come true) Citrus macrophylla ‘Alemow’ Most Oranges Citrus reshni ‘Cleopatra’ mandarin Changshou Kumquat Citrus sunki (Citrus reticulata var. austera) Sour mandarin Meiwa Kumquat (mostly polyembryonic) Citrus trifoliata (Poncirus trifoliata) Trifoliate orange Most Satsumas and Tangerines The following mandarin varieties are polyembryonic: Most Lemons Dancy Most Limes Emperor Grapefruits Empress Tangelos Fairchild Kinnow Highly monoembryonic citrus types: Mediterranean (Avana, Tardivo di Ciaculli) Will produce zygotic monoembryonic seeds that will not Naartje come true to type. -

Lemons & Limes
Reasons to Eat Lemons & Limes • Fresh lemon and lime pieces, and juice, have vitamin C, which keep your immune system healthy, preventing colds. • Lemons and limes also have fiber in them, which keeps your digestive system healthy. • Limes have iron in them, which keeps your muscles healthy. • Lemons and limes are low in saturated fat and low in sodium. www.sdharvestofthemonth.org Quick Serving Ideas • Place thinly sliced lemons, peel and all, Lemons & underneath and around fish before cooking. Baking or broiling will soften the slices so that they can be eaten along with the fish. Limes • Combine lemon juice with olive or flax oil, freshly crushed garlic and pepper to make a light and refreshing salad dressing. • To decrease salt consumption, serve lemon wedges with meals, as their tartness makes a great salt substitute. • Add an-easy-to-prepare zing to dinner tonight by tossing: seasoned cooked brown rice with garden peas, chicken pieces, scallions, pumpkin seeds, lime or lime juice and zest. • Squeeze some lime or lemon juice onto an avocado quarter and eat as is. How to Store and Juice Lemons & Limes • Lemons and limes can be stored at room temperature for up to one week. • They can be stored for two-three weeks in the refrigerator. • Lemons and limes are often called for in recipes in the form of juice. They will produce more juice when warmer, so always juice them at room temperature, or place them in a bowl of warm water. • Rolling them under the palm of your hand on a flat surface will also help to extract more juice. -
Holdings of the University of California Citrus Variety Collection 41
Holdings of the University of California Citrus Variety Collection Category Other identifiers CRC VI PI numbera Accession name or descriptionb numberc numberd Sourcee Datef 1. Citron and hybrid 0138-A Indian citron (ops) 539413 India 1912 0138-B Indian citron (ops) 539414 India 1912 0294 Ponderosa “lemon” (probable Citron ´ lemon hybrid) 409 539491 Fawcett’s #127, Florida collection 1914 0648 Orange-citron-hybrid 539238 Mr. Flippen, between Fullerton and Placentia CA 1915 0661 Indian sour citron (ops) (Zamburi) 31981 USDA, Chico Garden 1915 1795 Corsican citron 539415 W.T. Swingle, USDA 1924 2456 Citron or citron hybrid 539416 From CPB 1930 (Came in as Djerok which is Dutch word for “citrus” 2847 Yemen citron 105957 Bureau of Plant Introduction 3055 Bengal citron (ops) (citron hybrid?) 539417 Ed Pollock, NSW, Australia 1954 3174 Unnamed citron 230626 H. Chapot, Rabat, Morocco 1955 3190 Dabbe (ops) 539418 H. Chapot, Rabat, Morocco 1959 3241 Citrus megaloxycarpa (ops) (Bor-tenga) (hybrid) 539446 Fruit Research Station, Burnihat Assam, India 1957 3487 Kulu “lemon” (ops) 539207 A.G. Norman, Botanical Garden, Ann Arbor MI 1963 3518 Citron of Commerce (ops) 539419 John Carpenter, USDCS, Indio CA 1966 3519 Citron of Commerce (ops) 539420 John Carpenter, USDCS, Indio CA 1966 3520 Corsican citron (ops) 539421 John Carpenter, USDCS, Indio CA 1966 3521 Corsican citron (ops) 539422 John Carpenter, USDCS, Indio CA 1966 3522 Diamante citron (ops) 539423 John Carpenter, USDCS, Indio CA 1966 3523 Diamante citron (ops) 539424 John Carpenter, USDCS, Indio -

The Asian Citrus Psyllid and the Citrus Disease Huanglongbing
The Asian Citrus Psyllid and the Citrus Disease Huanglongbing Psyllid M. Rogers Beth Grafton-Cardwell Dept of Entomology, UC Riverside and Director Lindcove Research and Extension Center Huanglongbing It has an egg stage, 5 wingless intermediate stages called nymphs, and winged adults Adult The pest insect Egg 5 Nymphs (insects molt to grow bigger) Adult psyllids can feed on either young or mature leaves. This allows adults to survive year-round. The pest insect M. Rogers When feeding, the adult leans forward on its elbows and tips its rear end up in a very o M. Rogers characteristic 45 angle. The eggs are yellow-orange, tucked into the tips of tiny new leaves. They are difficult to see because they are so small The pest insect M. Rogers The nymphs produce waxy tubules that direct the honeydew away from their bodies. These tubules are unique and easy to recognize. Nymphs can only survive by living on young, tender The leaves and stems. pest insect M. Rogers Thus, nymphs are found only when the plant is producing new leaves. M. Rogers As the psyllid feeds, it injects a salivary toxin that causes the tips of new leaves to easily break off. If the leaf survives, then it twists as it grows. Twisted leaves can be a sign that the psyllid has been there. The pest insect M. Rogers M. Rogers M. Rogers What plants can the psyllid attack? All types of citrus and closely related plants in the Rutaceae family • Citrus (limes, lemons, oranges, grapefruit, mandarins…) • Fortunella (kumquats) • Citropsis (cherry orange) • Murraya paniculata (orange jasmine) • Bergera koenigii (Indian curry leaf) Plants • Severinia buxifolia (Chinese box orange) affected • Triphasia trifolia (limeberry) • Clausena indica (wampei) • Microcitrus papuana (desert-lime) • Others…. -

New and Noteworthy Citrus Varieties Presentation
New and Noteworthy Citrus Varieties Citrus species & Citrus Relatives Hundreds of varieties available. CITRON Citrus medica • The citron is believed to be one of the original kinds of citrus. • Trees are small and shrubby with an open growth habit. The new growth and flowers are flushed with purple and the trees are sensitive to frost. • Ethrog or Etrog citron is a variety of citron commonly used in the Jewish Feast of Tabernacles. The flesh is pale yellow and acidic, but not very juicy. The fruits hold well on the tree. The aromatic fruit is considerably larger than a lemon. • The yellow rind is glossy, thick and bumpy. Citron rind is traditionally candied for use in holiday fruitcake. Ethrog or Etrog citron CITRON Citrus medica • Buddha’s Hand or Fingered citron is a unique citrus grown mainly as a curiosity. The six to twelve inch fruits are apically split into a varying number of segments that are reminiscent of a human hand. • The rind is yellow and highly fragrant at maturity. The interior of the fruit is solid rind with no flesh or seeds. • Fingered citron fruits usually mature in late fall to early winter and hold moderately well on the tree, but not as well as other citron varieties. Buddha’s Hand or Fingered citron NAVEL ORANGES Citrus sinensis • ‘Washington navel orange’ is also known • ‘Lane Late Navel’ was the first of a as the Bahia. It was imported into the number of late maturing Australian United States in 1870. navel orange bud sport selections of Washington navel imported into • These exceptionally delicious, seedless, California. -

Additional Cocktail Recipes
Recipes From Philip Greene’s Smithsonian Associates Classic Summer Cocktails Seminar [email protected] Classic Highball 2 oz spirit 4 oz carbonated mixer Serve in a tall glass on ice. Garnish lemon or lime wedge/peel Moscow Mule 2 oz vodka ½ oz lime juice 4 oz ginger beer Add all ingredients to a copper mug (or rocks glass). Garnish with the lime hull. Audrey Saunders’ delicious riff on the Moscow Mule, the Gin-Gin Mule ¾ oz fresh lime juice 1 oz simple syrup 2 mint sprigs 1¾ oz Tanqueray London dry gin 1 oz homemade ginger beer* Shake all ingredients well, strain into a tall Collins glass filled with fresh ice. Garnish with a lime wheel, a sprig of mint and a piece of candied ginger. Serve with a straw. *Ginger Beer 1 gallon water 1 pound ginger root, peeled 4 oz light brown sugar or 6 oz light agave syrup 2 oz fresh lime juice Place the water in a sauce pan, bring to a boil. Cut the ginger into smaller pieces and mix this in a food processor with some of the hot water until the mixture resembles mulch. Then add this back into the pot of water, turn off the heat, cover, and allow it to steep for one hour. Once cooled, strain the mixture through cheese cloth or a sieve, extracting as much flavor from the solids as you can. Discard the solids. Add the lime juice and sugar/syrup, stir. Let cool, then transfer to bottle(s). Store in refrigerator. Note: this will not yield a conventional, carbonated ginger beer. -

Citrus Bacterial Canker Disease and Huanglongbing (Citrus Greening)
PUBLICATION 8218 Citrus Bacterial Canker Disease and Huanglongbing (Citrus Greening) MARYLOU POLEK, Program Manager and Plant Pathologist, Citrus Tristeza Virus Program, California Department of Food and Agriculture, Tulare; GEORGIOS VIDALAKIS, Director, Citrus Clonal Protection Program (CCPP), Department of Plant Pathology, University of UNIVERSITY OF California, Riverside; and KRIS GODFREY, Senior Environmental Research Scientist, CALIFORNIA Biological Control Program, California Department of Food and Agriculture, Sacramento Division of Agriculture and Natural Resources INTroduCTioN http://anrcatalog.ucdavis.edu Compared with the rest of the world, the California citrus industry is relatively free of diseases that can impact growers’ profits. Unfortunately, exotic plant pathogens may become well established before they are recognized as such. This is primarily because some of the initial symptoms mimic other diseases, mineral deficiencies, or toxicities. In addition, development of disease symptoms caused by some plant pathogenic organisms occurs a long time after initial infection. This long latent period results in significantly delayed disease diagnosis and pathogen detection. Citrus canker (CC) and huanglong- bing (HLB, or citrus greening) are two very serious diseases of citrus that occur in many other areas of the world but are not known to occur in California. However, if the patho- gens causing these diseases are introduced into California, they will create serious prob- lems for the state’s citrus production and nursery industries. CiTrus BACTerial CaNker Disease Citrus bacterial canker disease (CC) is caused by pathotypes or variants of the bacterium Xanthomonas axonopodis (formerly campestris) pv. citri (Xac). This bacterium is a quar- antine pest for many citrus-growing countries and is strictly regulated by international phytosanitary programs. -

Grapefruit, Lemon, Lime and Orange MARIA CARVALHO, IS
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Biblioteca Digital do IPB Targeting excessive free radicals with peels and juices of citrus fruits: grapefruit, lemon, lime and orange RAFAELA GUIMARÃES, LILLIAN BARROS, JOÃO C.M. BARREIRA, Mª JOÃO SOUSA, ANA MARIA CARVALHO, ISABEL C.F.R. FERREIRA* CIMO/Escola Superior Agrária, Instituto Politécnico de Bragança, Campus de Santa Apolónia, Apartado 1172, 5301-855 Bragança, Portugal. * Author to whom correspondence should be addressed (e-mail: [email protected] telephone +351-273-303219; fax +351-273-325405). 1 ABSTRACT A comparative study between the antioxidant properties of peel (flavedo and albedo) and juice of some commercially grown citrus fruit (Rutaceae), grapefruit (Citrus paradisi), lemon (Citrus limon), lime (Citrus x aurantiifolia) and sweet orange (Citrus sinensis) was performed. Different in vitro assays were applied to the volatile and polar fractions of peels and to crude and polar fraction of juices: 2,2-diphenyl-1- picrylhydrazyl (DPPH) radical scavenging capacity, reducing power and inhibition of lipid peroxidation using β-carotene-linoleate model system in lipossomes and thiobarbituric acid reactive substances (TBARS) assay in brain homogenates. Reducing sugars and phenolics were the main antioxidant compounds found in all the extracts. Peels polar fractions revealed the highest contents in phenolics, flavonoids, ascorbic acid, carotenoids and reducing sugars, which certainly contribute to the highest antioxidant potential found in these fractions. Peels volatile fractions were clearly separated using discriminat analysis, which is in agreement with their lowest antioxidant potential. KEYWORDS: Citrus fruits; Antioxidants; Scavenging activity; Peroxidation inhibition. 2 1. -

Mexican Lime
MEXICAN LIME Commercial citrus production in the Yuma area is devoted primarily to tangelo/tangerines, a distant second to desert lemons, the major citrus fruit grown in the area. Mexican limes, however, fit into a local niche market, with the small acreage grown essentially for local markets. · Of the two acid, or sour, limes in world trade, the one longest known and most widely cultivated is the Mexican lime (Citrus aurantifoli), and many often refer the tangy fruit merely as "lime". · The Mexican lime tree is exceptionally vigorous; may be shrubby and range from 6 1/2 to 13 feet high, with many slender, spreading branches, and usually with numerous, very sharp spines to 3/8 inch long. The lime fruits are borne singly or in 2's or 3's (or sometimes large clusters), at the twig tips. The pulp of the Mexican lime is greenish-yellow and the fruits are quite juicy, very acid and flavorful, with few or many small seeds which are also green in color. · The Mexican lime, because of its special bouquet and unique flavor, is ideal for serving in half as a garnish and flavoring for fish and meats, for adding zest to cold drinks, and for making limeade. Commercially bottled lime juice is prized the world over for use in mixed alcoholic drinks. · Limes are a very juicy citrus fruit. In fact, it is calculated that 2,200 lbs of fruit yields 1,058 pounds of juice. · Mexican limes are often made into jam, jelly and marmalade. They are also pickled by first making 4 incisions at the top of the fruit and covering the fruits with salt, and later preserv- ing them in vinegar. -

Citrus Offers Year-Round Options
for the Gardener Citrus Offers Year-Round Options he genus Citrus is undoubtedly the premier genus in the Rutaceae (Rue) TABLE 1. GENUS CITRUS: PROMINENT SPECIES AND PLACES OF ORIGIN, FROM LEAST COLD HARDY TO MOST COLD HARDY family. This family features 150 genera T 32ºF* Citrus medica India Citron/Etrog, Buddha’s Hand Fruit and 1600 species and consists largely 32ºF C. aurantiifolia India, S.E. Asia Limes: Mexican, Bearss, of evergreen shrubs and trees from the Key Lime, Tahitian Lime Mediterranean, subtropical, semitropical 28ºF C. limon S.E. Asia Lemons or limonia and tropical zones of the world. Table 1 26ºF C. paradisi Carribean Grapefruit: natural hybrid between (at right) lists the prominent species of lemon and pummelo the genus Citrus (and Fortunella), their 24ºF C. maxima Malaysian peninsula Pummelo hardiness, and place of origin. Among or grandis the other significant food and ornamental 24ºF C. paradisi (grapefruit) x Tangelo: bred cross of grapefruit genera in the family are – reticulata (Mandarin) and Mandarin Choisya (Mock orange) 24ºF C. sinensis S. E. China, Vietnam Oranges: sweet, sour, blood 24ºF C. nobilis (King) Florida Oranges: Temple, sweet Correa (Coral bells) reticulata x sinensis Dictamnus (Burning bush) 22ºF C. reticulata S.E. Asia Mandarins, Tangerines Casimiroa (White zapote) 22ºF C. limon cv. ‘Improved Meyer Lemon’ Bred hybrid cross, orange x lemon Fortunella (Kumquats and their 20ºF C. mitis Phillipines Calamondin 18ºF Fortunella S.E. China Kumquat, Limequat, Orangequat, hybrid forms) -20ºF margarita and hybrids Citrangequat Poncirus (Trifoliate orange and * approximate temperature at which frost damage begins to occur (flowers are flying dragon) the most cold-sensitive, followed by fruit, leaves, and wood) Ruta (Rue) Members of the Rue family feature strong oil glands in both the leaves and fruit.