Grapefruit, Lemon, Lime and Orange MARIA CARVALHO, IS
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Spicy Grapefruit Margarita. the RECIPE
THE RECIPE spicy grapefruit margarita. By halfbakedharvest I swear I have found the perfect amount of tequila, to lime juice, to fruity mixer for margs prep time 10 minutes total time 10 minutes servings 6 drinks 4.47 from 39 votes calories 207 kcal INGREDIENTS BIG BATCH MARGARITAS 1 1/2 cups silver tequila 12 ounces 2 cups grapefruit juice 16 ounces 3/4 cups fresh lime juice 1/4 cup agave nectar may also use honey 1-2 whole jalapeños halved (depending on how spicy you want your drink) slices lime + grapefruit for serving TO MAKE JUST ONE DRINK 1 1/2 - 2 ounces silver tequila 2 1/2 ounces grapefruit juice 1 ounce fresh lime juice 1 tablespoon agave nectar or more to taste 1-2 slices jalapeno depending on how spicy you want your drink slices lime + grapefruit for serving SPICY CHILI SALT RIM 1 tablespoon chili powder 1 tablespoon kosher salt 2 teaspoons granulated sugar INSTRUCTIONS SPICY CHILI SALT RIM In a bowl, combine the chili power salt and sugar. Mix well to combine. Use this mixture to rim your glasses. BIG BATCH MARGARITA Combine all the ingredients in a pitcher and stir to combine. Keep chilled in the fridge for 1-3 hours. Taste the drink every hour to check for spiciness. The longer you leave the jalapeño in the drink the spicier the drink will become. Once the drink is spiced to your liking, remove the jalapeños. When ready to serve, salt the rims of 4-6 glasses. Add ice to each glass and pour the margaritas over the ice. -

New Citrus Varieties Fort Bend County Master Gardeners
New Citrus Varieties Fort Bend County Master Gardeners Fruit Tree Sale, February 9, 2019 Written by Deborah Birge, Fort Bend County Master Gardener Citrus is one of the most sought-after fruit trees for residents of Fort Bend County. Nothing is more intoxicating than a tree full of orange or lemon blossoms. Nothing more inviting than a tree full of tasty oranges or satsumas ready for the picking. Citrus can be cold hardy needing little winter care or very cold sensitive needing protection from temperatures below 40 degrees. Choosing the variety for your particular needs and interest can be accomplished by understanding the many varieties and their properties. Fort Bend County Master Gardeners will be offering several new citrus varieties at the Annual Fruit Tree Sale, February 9, 2019. We have a new pommelo, lime, three mandarins, and a grapefruit. This article will discuss each to help you in your decisions. Australian Finger Lime The Australian finger lime is described as Citrus Caviar. These small cucumber-shaped limes are practically in a category all their own. Their aromatic skin appears in a triad of colors and the flesh holds caviar- shaped vesicles that pop crisply in your mouth with an assertively tart punch. The tree is a small-leafed understory plant with some thorns. The tough climate conditions of the Australian coastal regions where Finger Limes were first grown make it suitable for a diversity of planting locations with little care or maintenance. It will even tolerate cooler weather down to a brief frost. Its compact, almost hedge-like stature make it an excellent candidate for tight spots in the garden or throughout the landscape. -

Lime (Citrus Aurantifolia (Christm.) Swingle) Essential Oils: Volatile Compounds, Antioxidant Capacity, and Hypolipidemic Effect
foods Article Lime (Citrus aurantifolia (Christm.) Swingle) Essential Oils: Volatile Compounds, Antioxidant Capacity, and Hypolipidemic Effect Li-Yun Lin 1,*, Cheng-Hung Chuang 2 , Hsin-Chun Chen 3 and Kai-Min Yang 4 1 Department of Food Science and Technology, Hungkuang University, Taichung 433, Taiwan 2 Department of Nutrition, Hungkuang University, Taichung 433, Taiwan 3 Department of Cosmeceutics, China Medical University, Taichung 404, Taiwan 4 Department of Hospitality Management, Mingdao Unicersity, ChangHua 523, Taiwan * Correspondence: [email protected]; Tel.: +886-33-432-793 Received: 19 July 2019; Accepted: 2 September 2019; Published: 7 September 2019 Abstract: Lime peels are mainly obtained from the byproducts of the juice manufacturing industry, which we obtained and used to extract essential oil (2.3%) in order to examine the antioxidant and hypolipidaemic effects. We identified 60 volatile compounds of lime essential oil (LEO) with GC/MS, of which the predominant constituents were limonene, γ-terpinene, and β-pinene. Lime essential oil was measured according to the DPPH assay and ABTS assay, with IC50 values of 2.36 mg/mL and 0.26 mg/mL, respectively. This study also explored the protective effects of LEO against lipid-induced hyperlipidemia in a rat model. Two groups of rats received oral LEO in doses of 0.74 g/100 g and 2.23 g/100 g with their diets. Eight weeks later, we found that the administration of LEO improved the serum total cholesterol, triglyceride, low-density lipoprotein cholesterol, alanine aminotransferase, and aspartate transaminase levels in the hyperlipidemic rats (p < 0.05). Simultaneously, the LEO improved the health of the rats in terms of obesity, atherogenic index, and fatty liver. -

Tropical Horticulture: Lecture 32 1
Tropical Horticulture: Lecture 32 Lecture 32 Citrus Citrus: Citrus spp., Rutaceae Citrus are subtropical, evergreen plants originating in southeast Asia and the Malay archipelago but the precise origins are obscure. There are about 1600 species in the subfamily Aurantioideae. The tribe Citreae has 13 genera, most of which are graft and cross compatible with the genus Citrus. There are some tropical species (pomelo). All Citrus combined are the most important fruit crop next to grape. 1 Tropical Horticulture: Lecture 32 The common features are a superior ovary on a raised disc, transparent (pellucid) dots on leaves, and the presence of aromatic oils in leaves and fruits. Citrus has increased in importance in the United States with the development of frozen concentrate which is much superior to canned citrus juice. Per-capita consumption in the US is extremely high. Citrus mitis (calamondin), a miniature orange, is widely grown as an ornamental house pot plant. History Citrus is first mentioned in Chinese literature in 2200 BCE. First citrus in Europe seems to have been the citron, a fruit which has religious significance in Jewish festivals. Mentioned in 310 BCE by Theophrastus. Lemons and limes and sour orange may have been mutations of the citron. The Romans grew sour orange and lemons in 50–100 CE; the first mention of sweet orange in Europe was made in 1400. Columbus brought citrus on his second voyage in 1493 and the first plantation started in Haiti. In 1565 the first citrus was brought to the US in Saint Augustine. 2 Tropical Horticulture: Lecture 32 Taxonomy Citrus classification based on morphology of mature fruit (e.g. -

Tangerines, Mandarins, Satsumas, and Tangelos
Tangerines, Mandarins, Satsumas, and Tangelos Category: Semi-evergreen Hardiness: Damage will occur when temperatures drop below the low 20’s Fruit Family: Citrus Light: Full sun to half day sun Size: 10’H x 10’W; may be pruned to desired HxW Soil: Well-drained Planting: Plant after danger of frost has passed, mid to late March The name “tangerine” derives from one variety that was imported to Europe from Tangiers. There are many named varieties of what citrus growers call “mandarins” because of their Asian origins. One of these, the “Satsuma”, is an heirloom Japanese mandarin that is both delicious and especially adapted to Southeast Texas. It has been part of Gulf Coast Citrus history for a century. There are many named varieties of Satsumas. Mandarins are mostly orange-fleshed, juicy, highly productive, very easy to care for, long-lived, easily peeled and segmented or juiced. Few fruits can match the mandarin. Satsumas are seedless or close to seedless. They are all of outstanding quality and differ little among themselves except for when they ripen. Buy early, mid and late season varieties to have months of ripe fruit harvests from September to April. Care of Mandarins and related fruits Planting: Newly purchased citrus have probably not been hardened off to tolerate our winter weather. Keep your citrus in the container until late March, or until all danger of freeze has passed. Trees can be kept outside in a sunny area on mild days and nights, but move them into the shelter of the garage or house if frost is predicted. -

Facts About Citrus Fruits and Juices: Grapefruit1 Gail C
Archival copy: for current recommendations see http://edis.ifas.ufl.edu or your local extension office. FSHN02-6 Facts About Citrus Fruits and Juices: Grapefruit1 Gail C. Rampersaud2 Grapefruit is a medium- to large-sized citrus fruit. It is larger than most oranges and the fruit may be flattened at both ends. The skin is mostly yellow but may include shades of green, white, or pink. Skin color is not a sign of ripeness. Grapefruit are fully ripe when picked. Popular varieties of Florida grapefruit include: Did you know… Marsh White - white to amber colored flesh and almost seedless. Grapefruit was first Ruby Red - pink to reddish colored flesh with few seeds. discovered in the West Flame - red flesh and mostly seedless. Indies and introduced to Florida in the 1820s. Most grapefruit in the U.S. is still grown in Florida. Compared to most citrus fruits, grapefruit have an extended growing season and several Florida Grapefruit got its name because it grows in varieties grow from September through June. clusters on the tree, just like grapes! Fresh citrus can be stored in any cool, dry place but will last longer if stored in the refrigerator. Do Imposter!! not store fresh grapefruit in plastic bags or film- wrapped trays since this may cause mold to grow on the fruit. Whether you choose white or pink grapefruit or grapefruit juice, you’ll get great taste and a variety of health benefits! Read on…. 1. This document is FSHN026, one of a series of the Food Science and Human Nutrition Department, Florida Cooperative Extension Service, Institute of Food and Agricultural Sciences, University of Florida. -
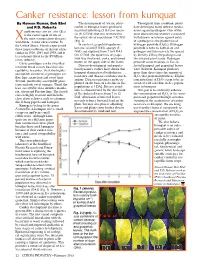
Canker Resistance: Lesson from Kumquat by Naveen Kumar, Bob Ebel the Development of Asiatic Citrus Throughout Their Evolution, Plants and P.D
Canker resistance: lesson from kumquat By Naveen Kumar, Bob Ebel The development of Asiatic citrus Throughout their evolution, plants and P.D. Roberts canker in kumquat leaves produced have developed many defense mecha- anthomonas citri pv. citri (Xcc) localized yellowing (5 DAI) or necro- nisms against pathogens. One of the is the causal agent of one of sis (9-12 DAI) that was restricted to most characteristic features associated the most serious citrus diseases the actual site of inoculation 7-12 DAI with disease resistance against entry X (Fig. 2). of a pathogen is the production of worldwide, Asiatic citrus canker. In the United States, Florida experienced In contrast, grapefruit epidermis hydrogen peroxide (H2O2). Hydrogen three major outbreaks of Asiatic citrus became raised (5 DAI), spongy (5 peroxide is toxic to both plant and canker in 1910, 1984 and 1995, and it DAI) and ruptured from 7 to 8 DAI. pathogen and thus restricts the spread is a constant threat to the $9 billion On 12 DAI, the epidermis of grape- by directly killing the pathogen and citrus industry. fruit was thickened, corky, and turned the infected plant tissue. Hydrogen Citrus genotypes can be classified brown on the upper side of the leaves. peroxide concentrations in Xcc-in- into four broad classes based on sus- Disease development and popula- fected kumquat and grapefruit leaves ceptibility to canker. First, the highly- tion dynamics studies have shown that were different. Kumquat produces susceptible commercial genotypes are kumquat demonstrated both disease more than three times the amount of Key lime, grapefruit and sweet lime. -

Citrus Varieties in Egypt: an Impression
International Research Journal of Applied Sciences Short Communication pISSN: 2663-5577, eISSN: 2663-5585 Citrus Varieties in Egypt: An Impression Waleed Fouad Abobatta Department of Citrus, Horticulture Research Institute, Agriculture Research Center, Egypt ARTICLE INFORMATION ABSTRACT Received: Citrus industry is very important for Egyptian economy, citrus fruit is the leading exportable agricultural product of Egypt and is an important source of national income. Citrus cultivation Accepted: area represents about 29% of the total fruit area, there are different citrus varieties cultivated in Egypt. This work aims to provide a short description of main citrus varieties cultivated in Published: Egypt through providing information about fruit size, maturity periods, seediness and productivity average and main cultivated areas for each variety. However Washington Navel Corresponding Author: and Valencia orange are the main varieties followed by Mandarins group varieties, lemon, Waleed Fouad Abobatta, Balady orange, while other varieties like Grapefruit, Sour orange and Kumquat are cultivated Department of Citrus, in small areas. Horticulture Research Institute, Agriculture Research Center, Egypt Key words: Citrus industry, navel orange, valencia orange, mandarins group, citrus varieties INTRODUCTION Citrus is a genus from Rutaceae family, subfamily Aurantoideae1 and there are several species in this genus; but there are major species such as sweet orange (Citrus sinensis (L.) Osbeck), mandarins group, grapefruits (Citrus paradisi ), lime (Citrus aurantifolia) and sour orange (Citrus aurantium L.)2. Citrus is a diploid genus origin in tropical, subtropical, but now it is produced mainly in arid and semiarid regions. Citrus species are among the most widely grown fruit crops in the world and have a huge market all over the world3. -

Citrus from Seed?
Which citrus fruits will come true to type Orogrande, Tomatera, Fina, Nour, Hernandina, Clementard.) from seed? Ellendale Tom McClendon writes in Hardy Citrus Encore for the South East: Fortune Fremont (50% monoembryonic) “Most common citrus such as oranges, Temple grapefruit, lemons and most mandarins Ugli Umatilla are polyembryonic and will come true to Wilking type. Because most citrus have this trait, Highly polyembryonic citrus types : will mostly hybridization can be very difficult to produce nucellar polyembryonic seeds that will grow true to type. achieve…. This unique characteristic Citrus × aurantiifolia Mexican lime (Key lime, West allows amateurs to grow citrus from seed, Indian lime) something you can’t do with, say, Citrus × insitorum (×Citroncirus webberii) Citranges, such as Rusk, Troyer etc. apples.” [12*] Citrus × jambhiri ‘Rough lemon’, ‘Rangpur’ lime, ‘Otaheite’ lime Monoembryonic (don’t come true) Citrus × limettioides Palestine lime (Indian sweet lime) Citrus × microcarpa ‘Calamondin’ Meyer Lemon Citrus × paradisi Grapefruit (Marsh, Star Ruby, Nagami Kumquat Redblush, Chironja, Smooth Flat Seville) Marumi Kumquat Citrus × sinensis Sweet oranges (Blonde, navel and Pummelos blood oranges) Temple Tangor Citrus amblycarpa 'Nasnaran' mandarin Clementine Mandarin Citrus depressa ‘Shekwasha’ mandarin Citrus karna ‘Karna’, ‘Khatta’ Poncirus Trifoliata Citrus kinokuni ‘Kishu mandarin’ Citrus lycopersicaeformis ‘Kokni’ or ‘Monkey mandarin’ Polyembryonic (come true) Citrus macrophylla ‘Alemow’ Most Oranges Citrus reshni ‘Cleopatra’ mandarin Changshou Kumquat Citrus sunki (Citrus reticulata var. austera) Sour mandarin Meiwa Kumquat (mostly polyembryonic) Citrus trifoliata (Poncirus trifoliata) Trifoliate orange Most Satsumas and Tangerines The following mandarin varieties are polyembryonic: Most Lemons Dancy Most Limes Emperor Grapefruits Empress Tangelos Fairchild Kinnow Highly monoembryonic citrus types: Mediterranean (Avana, Tardivo di Ciaculli) Will produce zygotic monoembryonic seeds that will not Naartje come true to type. -

Citrus Fruits 2020 Summary (August 2020) 3 USDA, National Agricultural Statistics Service
United States Department of Citrus Fruits Agriculture National 2020 Summary Agricultural Statistics Service August 2020 ISSN: 1948-9048 Contents Utilized Citrus Production – United States Chart ................................................................................................................... 6 Citrus Value of Production – United States Chart .................................................................................................................. 6 Citrus Narrative ....................................................................................................................................................................... 7 Citrus Acreage, Production, Utilization, and Value – States and United States: 2017-2018, 2018-2019, and 2019-2020 ........................................................................................................................................................................ 8 Citrus Acreage, Production, Utilization, and Value by Crop – United States: 2017-2018, 2018-2019, and 2019-2020 ........................................................................................................................................................................ 9 Orange Acreage, Yield, Utilization, Price, and Value by Type – States and United States: 2017-2018, 2018-2019, and 2019-2020 ................................................................................................................................................... 10 Bearing Acres of Oranges – United States Chart ................................................................................................................. -

Lemons & Limes
Reasons to Eat Lemons & Limes • Fresh lemon and lime pieces, and juice, have vitamin C, which keep your immune system healthy, preventing colds. • Lemons and limes also have fiber in them, which keeps your digestive system healthy. • Limes have iron in them, which keeps your muscles healthy. • Lemons and limes are low in saturated fat and low in sodium. www.sdharvestofthemonth.org Quick Serving Ideas • Place thinly sliced lemons, peel and all, Lemons & underneath and around fish before cooking. Baking or broiling will soften the slices so that they can be eaten along with the fish. Limes • Combine lemon juice with olive or flax oil, freshly crushed garlic and pepper to make a light and refreshing salad dressing. • To decrease salt consumption, serve lemon wedges with meals, as their tartness makes a great salt substitute. • Add an-easy-to-prepare zing to dinner tonight by tossing: seasoned cooked brown rice with garden peas, chicken pieces, scallions, pumpkin seeds, lime or lime juice and zest. • Squeeze some lime or lemon juice onto an avocado quarter and eat as is. How to Store and Juice Lemons & Limes • Lemons and limes can be stored at room temperature for up to one week. • They can be stored for two-three weeks in the refrigerator. • Lemons and limes are often called for in recipes in the form of juice. They will produce more juice when warmer, so always juice them at room temperature, or place them in a bowl of warm water. • Rolling them under the palm of your hand on a flat surface will also help to extract more juice. -

Pomelo & Grapefruit Imports & Exports
2000-2011 French grapefruit imports, 2000-2011 French pomelo-grapefruit exports Page 1 sur 2 NOVAGRIM Fresh Fruit Importer & Vegetable Supplier since 1999 NOVAGRIM HOMEPAGE ENGLISH FRANCAIS POMELO & GRAPEFRUIT ESPAÑOL IMPORTS & EXPORTS TÜRKCE Follow @NOVAGRIM_FRANCE STATISTICAL DATA FRENCH FRUIT IMPORTS Apple imports Apricot imports Avocado imports Cherry imports Clementine & Mandarin Fig imports Grapefruit & Pomelo European pomelo and grapefruit imports have risen by 25 per cent between 2001 and 2010.The EU countries imported 508 716 MT of grapefruits and pomelos Kiwi fruit imports in 2001. This import volume reached a total of 633 379 MT in 2010 and 580 015 MT in 2011. France is the second largest grapefruit importer among the EU-27 countries, Netherlands being the biggest importer. France and Netherlands imported 42 per cent of the total European Union grapefruit imports in 2010. While Lemon & Lime imports France imported 75 588 MT of grapefruits and pomelos in 2011 Netherlands imported 173 562 MT. Mango imports By the 1st of June 2012 ( January to June ) , France has already imported 34 888 MT of grapefuits and pomelos, mostly from Spain (12 151 MT), United States (8 612 MT), Israel (7 726 MT), Turkey (2 046 MT), China (1 369 MT) and South Africa (1 022 MT). Orange imports Peach & Nectarine imports France and Netherlands imported about 43 per cent of the total European Union grapefruit imports in 2012. While France imported 77 292 MT of grapefruits and pomelos in 2012 Netherlands imported 167 534 MT. The third largest grapefruit importer of 2012 has been Germany with a total of 55 567 MT of grapefruit imports.