Menlo Therapeutics Inc. June 2019
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

3628-3641-Pruritus in Selected Dermatoses
Eur opean Rev iew for Med ical and Pharmacol ogical Sci ences 2016; 20: 3628-3641 Pruritus in selected dermatoses K. OLEK-HRAB 1, M. HRAB 2, J. SZYFTER-HARRIS 1, Z. ADAMSKI 1 1Department of Dermatology, University of Medical Sciences, Poznan, Poland 2Department of Urology, University of Medical Sciences, Poznan, Poland Abstract. – Pruritus is a natural defence mech - logical self-defence mechanism similar to other anism of the body and creates the scratch reflex skin sensations, such as touch, pain, vibration, as a defensive reaction to potentially dangerous cold or heat, enabling the protection of the skin environmental factors. Together with pain, pruritus from external factors. Pruritus is a frequent is a type of superficial sensory experience. Pruri - symptom associated with dermatoses and various tus is a symptom often experienced both in 1 healthy subjects and in those who have symptoms systemic diseases . Acute pruritus often develops of a disease. In dermatology, pruritus is a frequent simultaneously with urticarial symptoms or as an symptom associated with a number of dermatoses acute undesirable reaction to drugs. The treat - and is sometimes an auxiliary factor in the diag - ment of this form of pruritus is much easier. nostic process. Apart from histamine, the most The chronic pruritus that often develops in pa - popular pruritus mediators include tryptase, en - tients with cholestasis, kidney diseases or skin dothelins, substance P, bradykinin, prostaglandins diseases (e.g. atopic dermatitis) is often more dif - and acetylcholine. The group of atopic diseases is 2,3 characterized by the presence of very persistent ficult to treat . Persistent rubbing, scratching or pruritus. -
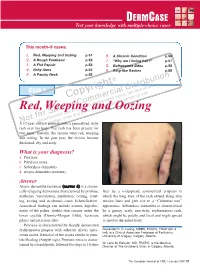
Red, Weeping and Oozing P.51 6
DERM CASE Test your knowledge with multiple-choice cases This month–9 cases: 1. Red, Weeping and Oozing p.51 6. A Chronic Condition p.56 2. A Rough Forehead p.52 7. “Why am I losing hair?” p.57 3. A Flat Papule p.53 8. Bothersome Bites p.58 4. Itchy Arms p.54 9. Ring-like Rashes p.59 5. A Patchy Neck p.55 on © buti t ri , h ist oad rig D wnl Case 1 y al n do p ci ca use o er sers nal C m d u rso m rise r pe o utho y fo C d. A cop or bite ngle Red, Weleepirnohig a sind Oozing a se p rint r S ed u nd p o oris w a t f uth , vie o Una lay AN12-year-old boy dpriesspents with a generalized, itchy rash over his body. The rash has been present for two years. Initially, the lesions were red, weeping and oozing. In the past year, the lesions became thickened, dry and scaly. What is your diagnosis? a. Psoriasis b. Pityriasis rosea c. Seborrheic dermatitis d. Atopic dermatitis (eczema) Answer Atopic dermatitis (eczema) (answer d) is a chroni - cally relapsing dermatosis characterized by pruritus, later by a widespread, symmetrical eruption in erythema, vesiculation, papulation, oozing, crust - which the long axes of the rash extend along skin ing, scaling and, in chronic cases, lichenification. tension lines and give rise to a “Christmas tree” Associated findings can include xerosis, hyperlin - appearance. Seborrheic dermatitis is characterized earity of the palms, double skin creases under the by a greasy, scaly, non-itchy, erythematous rash, lower eyelids (Dennie-Morgan folds), keratosis which might be patchy and focal and might spread pilaris and pityriasis alba. -

Photodermatoses Update Knowledge and Treatment of Photodermatoses Discuss Vitamin D Levels in Photodermatoses
Ashley Feneran, DO Jenifer Lloyd, DO University Hospitals Regional Hospitals AMERICAN OSTEOPATHIC COLLEGE OF DERMATOLOGY Objectives Review key points of several photodermatoses Update knowledge and treatment of photodermatoses Discuss vitamin D levels in photodermatoses Types of photodermatoses Immunologically mediated disorders Defective DNA repair disorders Photoaggravated dermatoses Chemical- and drug-induced photosensitivity Types of photodermatoses Immunologically mediated disorders Polymorphous light eruption Actinic prurigo Hydroa vacciniforme Chronic actinic dermatitis Solar urticaria Polymorphous light eruption (PMLE) Most common form of idiopathic photodermatitis Possibly due to delayed-type hypersensitivity reaction to an endogenous cutaneous photo- induced antigen Presents within minutes to hours of UV exposure and lasts several days Pathology Superficial and deep lymphocytic infiltrate Marked papillary dermal edema PMLE Treatment Topical or oral corticosteroids High SPF Restriction of UV exposure Hardening – natural, NBUVB, PUVA Antimalarial PMLE updates Study suggests topical vitamin D analogue used prophylactically may provide therapeutic benefit in PMLE Gruber-Wackernagel A, Bambach FJ, Legat A, et al. Br J Dermatol, 2011. PMLE updates Study seeks to further elucidate the pathogenesis of PMLE Found a decrease in Langerhans cells and an increase in mast cell density in lesional skin Wolf P, Gruber-Wackernagel A, Bambach I, et al. Exp Dermatol, 2014. Actinic prurigo Similar to PMLE Common in native -

Module Test № 1 on Dermatology
THE MINISTRY OF HEALTHCARE OF THE RUSSIAN FEDERATION FEDERAL STATE BUDGETARY EDUCATIONAL INSTITUTION OF HIGHER PROFESSIONAL EDUCATION PIROGOV RUSSIAN NATIONAL RESEARCH MEDICAL UNIVERSITY DEPARTMENT OF DERMATOVENEROLOGY Gaydina T.A., Dvornikov A.S., Skripkina P.A., Nazhmutdinova D.K., Heydar S.A., Arutunyan G.B., Pashinyan A.G. MODULE TEST №1 ON DERMATOLOGY FOR STUDENTS OF INSTITUTES OF HIGHER MEDICAL EDUCATION ON SPECIALTY THERAPEUTIC FACULTY DEPARTMENT OF DERMATOVENEROLOGY Moscow 2016 ISBN УДК ББК A21 Module test №1 on Dermatology for students of institutes of high medical education on specialty «Therapeutic faculty» department of dermatovenerology: manual for students for self-training//FSBEI HPE “Pirogov RNRMU” of the ministry of healthcare of the russian federation, M.: (publisher) 2016, 144 p. The manual is a part of teaching-methods on Dermatovenerology. It contains tests on Dermatology on the topics of practical sessions requiring single or multiple choice anser. The manual can be used to develop skills of students during practical sessions. It also can be used in the electronic version at testing for knowledge. The manual is compiled according to FSES on specialty “therapeutic faculty”, working programs on dermatovenerology. The manual is intended for foreign students of 3-4 courses on specialty “therapeutic faculty” and physicians for professional retraining. Authors: Gaydina T.A. – candidate of medical science, assistant of dermatovenerology department of therapeutic faculty Pirogov RNRMU Dvornikov A.S. – M.D., professor of dermatovenerology department of therapeutic faculty Pirogov RNRMU Skripkina P.A. – candidate of medical science, assistant professor of dermatovenerology department of therapeutic faculty Pirogov RNRMU Nazhmutdinova D.K. – candidate of medical science, assistant professor of dermatovenerology department of therapeutic faculty Pirogov RNRMU Heydar S.A. -

Update of the Guideline on Chronic Pruritus
Update of the Guideline on Chronic Pruritus Developed by the Guideline Subcommittee “Chronic Pruritus” of the European Dermatology Forum Subcommittee Members: Prof. Dr. Elke Weisshaar, Heidelberg (Germany) Prof. Dr. Sonja Ständer, Münster (Germany) Prof. Dr. Erwin Tschachler, Wien (Austria) Prof. Dr. Torello Lotti, Florence (Italy) Prof. Dr. Johannes Ring, Munich (Germany) Prof. Dr. Laurent Misery, Brest (France) Dr. Markus Streit, Aarau (Switzerland) Prof. Dr. Thomas Mettang, Wiesbaden (Germany) Prof. Dr. Jacek Szepietowski, Wroclaw (Poland) Prof. Dr. Joanna Wallengren, Lund (Sweden) Dr. Peter Maisel, Münster (Germany) Prof. Dr. Uwe Gieler, Gießen (Germany) Prof. Dr. Malcolm Greaves (Singapore) Prof. Dr. Ulf Darsow, Munich (Germany) Prof. Dr. Julien Lambert, Antwerp (Belgium) Members of EDF Guideline Committee: Prof. Dr. Werner Aberer, Graz (Austria) Prof. Dr. Dieter Metze, Münster (Germany) Prof. Dr. Martine Bagot, Paris (France) Dr. Kai Munte, Rotterdam (Netherlands) Prof. Dr. Nicole Basset-Seguin, Paris (France) Prof. Dr. Gilian Murphy, Dublin (Ireland) Prof. Dr. Ulrike Blume-Peytavi, Berlin (Germany) Prof. Dr. Martino Neumann, Rotterdam (Netherlands) Prof. Dr. Lasse Braathen, Bern (Switzerland) Prof. Dr. Tony Ormerod, Aberdeen (UK) Prof. Dr. Sergio Chimenti, Rome (Italy) Prof. Dr. Mauro Picardo, Rome (Italy) Prof. Dr. Alexander Enk, Heidelberg (Germany) Prof. Dr. Johannes Ring, Munich (Germany) Prof. Dr. Claudio Feliciani, Rome (Italy) Prof. Dr. Annamari Ranki, Helsinki (Finland) Prof. Dr. Claus Garbe, Tübingen (Germany) Prof. Dr. Berthold Rzany, Berlin (Germany) Prof. Dr. Harald Gollnick, Magdeburg (Germany) Prof. Dr. Rudolf Stadler, Minden (Germany) Prof. Dr. Gerd Gross, Rostock (Germany) Prof. Dr. Sonja Ständer, Münster (Germany) Prof. Dr. Vladimir Hegyi, Bratislava (Slovakia) Prof. Dr. Eggert Stockfleth, Berlin (Germany) Prof. Dr. -

Frontiers in Dermatology and Venereology - a Series of Theme Issues in Relation to the 100-Year Anniversary of Actadv
ISSN 0001-5555 ActaDV Volume 100 2020 Theme issue ADVANCES IN DERMATOLOGY AND VENEREOLOGY A Non-profit International Journal for Interdisciplinary Skin Research, Clinical and Experimental Dermatology and Sexually Transmitted Diseases Frontiers in Dermatology and Venereology - A series of theme issues in relation to the 100-year anniversary of ActaDV Official Journal of - European Society for Dermatology and Psychiatry Affiliated with - The International Forum for the Study of Itch Immediate Open Access Acta Dermato-Venereologica www.medicaljournals.se/adv ACTA DERMATO-VENEREOLOGICA The journal was founded in 1920 by Professor Johan Almkvist. Since 1969 ownership has been vested in the Society for Publication of Acta Dermato-Venereologica, a non-profit organization. Since 2006 the journal is published online, independently without a commercial publisher. (For further information please see the journal’s website https://www. medicaljournals.se/acta) ActaDV is a journal for clinical and experimental research in the field of dermatology and venereology and publishes high- quality papers in English dealing with new observations on basic dermatological and venereological research, as well as clinical investigations. Each volume also features a number of review articles in special areas, as well as Correspondence to the Editor to stimulate debate. New books are also reviewed. The journal has rapid publication times. Editor-in-Chief: Olle Larkö, MD, PhD, Gothenburg Former Editors: Johan Almkvist 1920–1935 Deputy Editors: Sven Hellerström 1935–1969 -

Abstracts from the 9Th World Congress on Itch October 15–17, 2017
ISSN 0001-5555 ActaDV Volume 97 2017 SEPTEMBER, No. 8 ADVANCES IN DERMATOLOGY AND VENEREOLOGY A Non-profit International Journal for Interdisciplinary Skin Research, Clinical and Experimental Dermatology and Sexually Transmitted Diseases Abstracts from the 9th World Congress on Itch October 15–17, 2017 Official Journal of - European Society for Dermatology and Wroclaw, Poland Psychiatry Affiliated with - The International Forum for the Study of Itch Immediate Open Access Acta Dermato-Venereologica www.medicaljournals.se/adv Abstracts from the 9th World Congress on Itch DV cta A enereologica V Organizing Committee Scientific Committee ermato- Congress President: Jacek C Szepietowski (Wroclaw, Poland) Jeffrey D. Bernhard (Massachusetts, USA) D Congress Secretary General: Adam Reich (Rzeszów, Poland) Earl Carstens (Davis, USA) Congress Secretary: Edyta Lelonek (Wroclaw, Poland) Toshiya Ebata (Tokyo, Japan) cta Alan Fleischer (Lexington, USA) A IFSI President: Earl Carstens (Davis, USA) IFSI Vice President: Elke Weisshaar (Heidelberg, Germany) Ichiro Katayama (Osaka, Japan) Ethan Lerner (Boston, USA) Staff members of the Department of Dermatology, Venereology and Allergology, Wroclaw Medical University, Wroclaw, Poland Thomas Mettang (Wiesbaden, Germany) Martin Schmelz (Mannheim, Germany) Sonja Ständer (Münster, Germany) DV Jacek C. Szepietowski (Wroclaw, Poland) cta Kenji Takamori (Tokyo, Japan) A Elke Weisshaar (Heidelberg, Germany) Gil Yosipovitch (Miami, USA) Contents of this Abstract book Program 1000 Abstracts: Lecture Abstracts 1008 Poster Abstracts 1035 Author Index 1059 dvances in dermatology and venereology A www.medicaljournals.se/acta doi: 10.2340/00015555-2773 Journal Compilation © 2017 Acta Dermato-Venereologica. Acta Derm Venereol 2017; 97: 999–1060 1000 9th World Congress of Itch Sunday, October 15, 2017 Abstract # 1:30-3:30 PM IFSI Board meeting DV 5:00-5:20 PM OPENING CEREMONY 5:00-5:10 PM Opening remarks cta Jacek C. -

Dermatological Disorders in Tuvalu Between 2009 and 2012
MOLECULAR MEDICINE REPORTS 12: 3629-3631, 2015 Dermatological disorders in Tuvalu between 2009 and 2012 LI-JUNG LAN1,2, YING-SHUANG LIEN2,3, SHAO-CHUAN WANG2,4, NESE ITUASO-CONWAY5, MING-CHE TSAI2,6, PAO-YING TSENG6, YU-LIN YEH1, CHUN-TZU CHEN7, KO‑HUANG LUE2,8, JING-GUNG CHUNG9,10 and YU-PING HSIAO1,2 1Department of Dermatology, Chung Shan Medical University Hospital, Taichung 40201; 2Institute of Medicine, School of Medicine, Chung Shan Medical University, Taichung 40201; Departments of 3Obstetrics and Gynecology, and 4Urology, Chung Shan Medical University Hospital, Taichung 40201; 5Public Health, Princess Margaret Hospital, Ministry of Health, Funafuti, Tuvalu; 6International Medical Service Center, Chung Shan Medical University Hospital, Taichung 40201; Departments of 7Chief Secretary Group and 8Pediatrics, Chung Shan Medical University Hospital, Taichung 40201; 9Department of Biological Science and Technology, China Medical University, Taichung 40201; 10Department of Biotechnology, Asia University, Taichung 41354, Taiwan, R.O.C. Received July 1, 2014; Accepted September 18, 2014 DOI: 10.3892/mmr.2015.3806 Abstract. There is a distinct lack of knowledge on the land area of Tuvalu is only 25.9 km2, and the highest point does prevalence of skin disorders in Tuvalu. The aim of the current not exceed 4 m above sea level (1). Currently, an estimated study was to assess the prevalence of cutaneous diseases and 11,000 people live in Tuvalu (1). The Taiwan International to evaluate access dermatological care in Tuvalu. Cutaneous Cooperation and Development Fund (Taiwan ICDF) has disorders in the people of Tuvalu between 2009 and 2012 coordinated the medical resources of Taiwanese hospitals in were examined. -

Clinical Spectrum of Ageing Versus Non-Ageing Geriatric Dermatoses -A Case-Controlled Study in Tertiary Care Centre, Tamil Nadu, South India
IP Indian Journal of Clinical and Experimental Dermatology 2020;6(2):151–159 Content available at: iponlinejournal.com IP Indian Journal of Clinical and Experimental Dermatology Journal homepage: www.innovativepublication.com Original Research Article Clinical spectrum of ageing versus non-ageing geriatric dermatoses -A case-controlled study in tertiary care centre, Tamil Nadu, South India C Abhirami1, P K Kaviarasan1,*, B Poorana1, K Kannambal1, P V S Prasad1, N Chidambaram2 1Dept. of Dermatology Venereology & Leprosy, Rajah Muthiah Medical College and Hospital, Annamalai University, Chidambaram, Tamil Nadu, India 2Dept. of Medicine, Rajah Muthiah Medical College and Hospital, Annamalai University, Chidambaram, Tamil Nadu, India ARTICLEINFO ABSTRACT Article history: Introduction: Ageing process is a complex phenomenon which affects all organs in the body as well as Received 17-05-2020 skin. Geriatric dermatoses can be regarded as a cutaneous marker of underlying systemic disorders and Accepted 29-05-2020 internal malignancies. This was study aimed to analyse various clinical ageing patterns among elderly aged Available online 25-06-2020 above 60 years. Objectives: To determine the prevalence of clinical patterns of ageing and non-ageing dermatoses among elderly compare with non-dermatology patients. Keywords: Materials and Methods: An observational study, conducted on 300 patients aged 60 years and above were Ageing analysed for geriatric dermatoses. Of them 211 patients were subjects attended dermatology OPD (group Dermatoses A) and 89 patients were controls (Group B) from other were selected from specialties.. Detailed history, Geriatric through examination and appropriate investigations were done after obtaining informed consent. Pruritus Ageing Results: Out of 300 patients, males were (192, 64%) and females (108, 36%), the male female ratio of Pruritus 1.77:1. -

Pathophysiology and Treatment of Pruritus in Elderly
International Journal of Molecular Sciences Review Pathophysiology and Treatment of Pruritus in Elderly Bo Young Chung † , Ji Young Um †, Jin Cheol Kim , Seok Young Kang , Chun Wook Park and Hye One Kim * Department of Dermatology, Kangnam Sacred Heart Hospital, Hallym University, Seoul KS013, Korea; [email protected] (B.Y.C.); [email protected] (J.Y.U.); [email protected] (J.C.K.); [email protected] (S.Y.K.); [email protected] (C.W.P.) * Correspondence: [email protected] † These authors contributed equally to this work. Abstract: Pruritus is a relatively common symptom that anyone can experience at any point in their life and is more common in the elderly. Pruritus in elderly can be defined as chronic pruritus in a person over 65 years old. The pathophysiology of pruritus in elderly is still unclear, and the quality of life is reduced. Generally, itch can be clinically classified into six types: Itch caused by systemic diseases, itch caused by skin diseases, neuropathic pruritus, psychogenic pruritus, pruritus with multiple factors, and from unknown causes. Senile pruritus can be defined as a chronic pruritus of unknown origin in elderly people. Various neuronal mediators, signaling mechanisms at neuronal terminals, central and peripheral neurotransmission pathways, and neuronal sensitizations are included in the processes causing itch. A variety of therapies are used and several novel drugs are being developed to relieve itch, including systemic and topical treatments. Keywords: elderly; ion channel; itch; neurotransmission pathophysiology of itch; pruritogen; senile pruritus; treatment of itch 1. Introduction Citation: Chung, B.Y.; Um, J.Y.; Kim, Pruritus is a relatively common symptom that anyone can experience at any point in J.C.; Kang, S.Y.; Park, C.W.; Kim, H.O. -

Guías Diagnósticas Y Terapeúticas De Las 10 Patologías Más Frecuentes
HOSPITAL INFANTIL DE MÉXICO “FEDERICO GÓMEZ” SERVICIO DE DERMATOLOGÍA GUÍAS DIAGNÓSTICAS Y TERAPÉUTICAS DE LAS 10 PATOLOGÍAS MÁS FRECUENTES DR CARLOS ALFREDO MENA CEDILLOS, JEFE DEL SERVICIO DRA ADRIANA MARÍA VALENCIA HERRERA DERMATITIS ATOPICA SINONIMIA. Neurodermatitis, prurigo de Besnier, eccema del lactante. DEFINICION. Enfermedad reaccional, crónica y recidivante de la piel, con un patrón clínico e historia natural característicos. No se conoce la causa específica, pero se ha relacionado con susceptibilidad genética, disturbios inmuológicos y constitucionales, sobre los que actúan factores desencadenantes. EPIDEMIOLOGIA: Es la dermatosis más frecuente en población pediátrica. La prevalencia ha mostrando incremento en las últimas décadas, siendo del 18-20%. Es mas frecuente en áreas urbanas de países industrializados, especialmente en inmigrantes provenientes de países con menor prevalencia. No existe clara predilección racial ni diferencia en cuanto al sexo. Puede presentarse a cualquier edad, con claro predominio en la población pediátrica, 60-85% de los casos inicia en el primer año de vida y 85-95% antes de los 5 años; 10-25% de los casos persiste con recaídas en la edad adulta. ETIOPATOGENIA. La etiología es desconocida pero parece ser resultado de una compleja interacción aspectos genéticos, inmunológicos y defectos en la barrera epidérmica, existiendo múltiples factores descencadenantes, queactúan sobre un terreno constitucionalmente alterado. 1. Anomalías genéticas. Tiene clara naturaleza familiar, pero no se ha precisado el mecanismo de herencia, existiendo en 70% de los pacientes antecedentes de atopia. Los antígenos de histocompatibilidad HL-A9, HL-A3, HL-B12 y HL-Bw40 se han descrito en estos pacientes. 2. Disturbios inmunológicos. Existen cambios significativos en la inmunidad humoral y celular. -

Chronic Pruritic Vulva Lesion
Photo RoUNDS Stephen Colden Cahill, DO Chronic pruritic vulva lesion College of Osteopathic Medicine, Michigan State University, East Lansing; The patient was not sexually active and denied any and Lakeshore Health vaginal discharge. So what was causing the intense Partners, Holland, Mich itching on her labia? [email protected] Department eDItOr richard p. Usatine, mD University of Texas Health Science Center at San Antonio During a routine exam, a 45-year-old Cau- both of her labia majora (FIGURE). Throughout casian woman complained of intense itching the lesion, there were scattered areas of exco- The author reported no potential conflict of interest on her labia. She said that the itching had been riation. Her labia minor were spared. relevant to this article. an issue for more than 9 months and that she A speculum and bimanual exam were found herself scratching several times a day. normal. No inguinal lymphadenopathy was She denied any vaginal discharge and said she present. hadn’t been sexually active in years. She had tried over-the-counter antifungals and topical ● WhaT is your diagnosis? Benadryl, but they provided only limited relief. The patient had red thickened plaques ● HoW Would you TREAT THIS with accentuated skin lines (furrows) covering PATIENT? Figure Red thickened plaques on labia majora p ho T o cour T esy of: St ephen c olden c ahill, DO jfponline.com Vol 62, no 2 | february 2013 | The journal of family pracTice 97 PHOTO RoUNDS Diagnosis: taneous lesion of unknown etiology. It can Lichen simplex chronicus be found throughout the body, including the This patient was given a diagnosis of lichen mucous membranes.